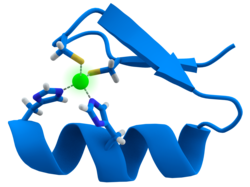
molecular biology, protein fold classes are broad categories of protein tertiary structure topology. They describe groups of proteins that share similar...
9 KB (961 words) - 18:18, 5 April 2025
Protein folding is the physical process by which a protein, after synthesis by a ribosome as a linear chain of amino acids, changes from an unstable random...
76 KB (8,708 words) - 09:25, 13 March 2025
a protein. For simple proteins, it can be the entire protein. The broadest groups on SCOP version 1.75 are the protein fold classes. These classes group...
22 KB (2,564 words) - 19:34, 16 August 2024
proteins. There are multiple fold classes of globular proteins, since there are many different architectures that can fold into a roughly spherical shape...
6 KB (746 words) - 12:06, 14 October 2024
Globin (redirect from Globin fold)
heme-containing globular proteins, involved in binding and/or transporting oxygen. These proteins all incorporate the globin fold, a series of eight alpha...
19 KB (2,067 words) - 22:39, 2 November 2024
AlphaFold is an artificial intelligence (AI) program developed by DeepMind, a subsidiary of Alphabet, which performs predictions of protein structure....
65 KB (5,831 words) - 16:10, 1 May 2025
Chaperonin (category Protein folding)
assisting protein folding. HSP60 belong to a large class of molecules that assist protein folding, called molecular chaperones. Newly made proteins usually...
22 KB (2,320 words) - 02:13, 5 May 2025
predict protein folding and thus protein structure, for example, Itasser, and AlphaFold. AlphaFold was one of the first AIs to predict protein structures....
74 KB (9,238 words) - 01:35, 3 April 2025
are proteins that assist the conformational folding or unfolding of large proteins or macromolecular protein complexes. There are a number of classes of...
29 KB (3,502 words) - 12:50, 3 March 2025
protein folding into a specific 3D structure that determines its activity. A linear chain of amino acid residues is called a polypeptide. A protein contains...
105 KB (10,957 words) - 19:34, 14 May 2025
Zinc finger (redirect from Zinc-finger protein)
finger is a small protein structural motif that is characterized by the coordination of one or more zinc ions (Zn2+) which stabilizes the fold. The term zinc...
42 KB (4,882 words) - 12:55, 7 February 2025
Membrane fusion proteins (not to be confused with chimeric or fusion proteins) are proteins that cause fusion of biological membranes. Membrane fusion...
17 KB (1,845 words) - 06:21, 9 January 2025
The jelly roll or Swiss roll fold is a protein fold or supersecondary structure composed of eight beta strands arranged in two four-stranded sheets. The...
27 KB (2,890 words) - 03:00, 6 January 2025
Small proteins are a diverse fold class of proteins (usually <100 amino acids long). Their tertiary structure is usually maintained by disulphide bridges...
6 KB (707 words) - 20:09, 12 May 2024
In molecular biology, a protein domain is a region of a protein's polypeptide chain that is self-stabilizing and that folds independently from the rest...
71 KB (8,443 words) - 19:00, 15 August 2024
helix/beta sheet proteins. While most proteins adopt a single stable fold, a few proteins can rapidly interconvert between one or more folds. These are referred...
12 KB (794 words) - 22:59, 14 April 2025
identified as a peptide, rather than a protein. To be able to perform their biological function, proteins fold into one or more specific spatial conformations...
38 KB (4,246 words) - 13:59, 17 January 2025
methods have a reasonable probability of predicting the folds of small, single-domain proteins within 1.5 angstroms over the entire structure. De novo...
23 KB (2,880 words) - 09:34, 19 February 2025
TIM barrel (category Protein folds)
enzymes adopting this fold. Further, five of seven enzyme commission (EC) enzyme classes include TIM barrel proteins. The TIM barrel fold is evolutionarily...
46 KB (4,821 words) - 15:00, 14 March 2025
Proteinopathy (redirect from Protein misfolding)
organs of the body. Often the proteins fail to fold into their normal configuration; in this misfolded state, the proteins can become toxic in some way...
62 KB (4,598 words) - 07:57, 20 April 2025
perform chaperone functions by stabilizing new proteins to ensure correct folding or by helping to refold proteins that were damaged by the cell stress. This...
49 KB (5,534 words) - 15:15, 2 May 2025
change can impact the protein's ability to function or to fold correctly. Misfolded proteins have a tendency to form dense protein clumps, which are often...
40 KB (4,406 words) - 01:13, 15 April 2025
help of an MHC class I protein. Because MHC class I molecules present peptides derived from cytosolic proteins, the pathway of MHC class I presentation...
23 KB (2,917 words) - 07:11, 19 April 2025
residues within proteins as they fold. This allows proteins to quickly find the correct arrangement of disulfide bonds in their fully folded state, and therefore...
19 KB (1,936 words) - 16:09, 10 July 2024
Proteostasis (redirect from Protein homeostasis)
pathways within cells that control the biogenesis, folding, trafficking, and degradation of proteins present within and outside the cell. Loss of proteostasis...
25 KB (2,922 words) - 18:48, 4 May 2025
CATH database (category Protein folds)
The CATH Protein Structure Classification database is a free, publicly available online resource that provides information on the evolutionary relationships...
7 KB (528 words) - 03:41, 31 March 2025
transcription, translation, post translational modifications, and protein folding. Proteins are made from amino acids. In humans, some amino acids can be...
27 KB (2,828 words) - 15:30, 9 January 2025
SH3 domain (category Protein domains)
The SH3-type fold is an ancient fold found in eukaryotes as well as prokaryotes. The classical SH3 domain is usually found in proteins that interact...
8 KB (933 words) - 12:56, 10 September 2024
multi-domain proteins. They are sometimes considered as a separate class of proteins along with globular, fibrous and membrane proteins. IDPs are a very...
54 KB (6,283 words) - 06:03, 7 April 2025
encode major capsid proteins that contain a single vertical jelly roll fold. All viruses in Singelaviria have two major capsid proteins (MCPs) that both...
17 KB (2,054 words) - 03:38, 14 March 2025