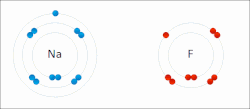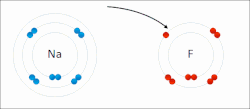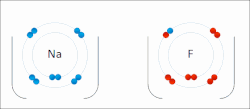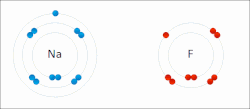
The electron affinity (Eea) of an atom or molecule is defined as the amount of energy released when an electron attaches to a neutral atom or molecule...
22 KB (1,468 words) - 11:02, 24 April 2025
electron affinity as a property of isolated atoms or molecules (i.e. in the gas phase). Solid state electron affinities are not listed here. Electron...
59 KB (4,158 words) - 14:51, 30 April 2025
Periodic table (section Electron affinity)
ionisation energy is the electron affinity, which is the energy released when adding an electron to the atom. A passing electron will be more readily attracted...
252 KB (27,179 words) - 14:30, 25 May 2025
Periodic trends (section Electron affinity)
Major periodic trends include atomic radius, ionization energy, electron affinity, electronegativity, nucleophilicity, electrophilicity, valency, nuclear...
19 KB (2,130 words) - 01:00, 31 January 2025
Anderson's rule (redirect from Electron affinity rule)
the electron affinity and band gap values for each semiconductor to calculate the conduction band and valence band offsets. The electron affinity (usually...
6 KB (788 words) - 06:16, 14 April 2025
correlated (for most and larger electronegativity values) with the electron affinity. It is to be expected that the electronegativity of an element will...
35 KB (4,372 words) - 07:58, 25 May 2025
the proton affinity is positive. This is the same sign convention used for electron affinity. The property related to the proton affinity is the gas-phase...
15 KB (1,028 words) - 15:15, 24 April 2024
systems with negative electron affinity and the emission from excited states, or a few hundred keV photons for core electrons in elements with a high...
59 KB (7,026 words) - 05:45, 23 May 2025
Koopmans' theorem (section For electron affinities)
statement of Koopmans' to calculate the electron affinity in this sense. Calculations of electron affinities using this statement of Koopmans' theorem...
24 KB (2,896 words) - 17:56, 24 May 2025
gained or lost, in an electron donor-acceptor transfer is determined by the difference between the acceptor's electron affinity (A) and the ionization...
5 KB (496 words) - 15:45, 20 January 2025
the electron affinity (note that this has a different meaning than the electron affinity of chemistry); in silicon for example the electron affinity is...
31 KB (3,661 words) - 10:21, 10 February 2025
have no electron affinity. Nevertheless, quantum electrodynamic corrections have been shown to be quite significant in reducing this affinity by decreasing...
64 KB (10,403 words) - 17:05, 24 May 2025
Electronegativity Electron affinity Étienne François Geoffroy — Geoffroy's 1718 Affinity Table Valency Affinity chromatography Affinity electrophoresis...
10 KB (1,341 words) - 19:24, 14 August 2024
{1}{2}}(I-A)\end{aligned}}} where I is the ionization potential and A the electron affinity. This expression implies that the chemical hardness is proportional...
19 KB (2,118 words) - 10:59, 24 May 2025
to remove the first electron from Mg, plus the ionization energy required to remove the second electron from Mg+. Electron affinity is defined as the amount...
7 KB (941 words) - 22:38, 25 March 2025
increases ununennium's electron affinity far beyond that of caesium and francium; indeed, ununennium is expected to have an electron affinity higher than all...
225 KB (24,165 words) - 17:24, 9 May 2025
Ununennium's electron affinity is expected to be far greater than that of caesium and francium; indeed, ununennium is expected to have an electron affinity higher...
46 KB (8,381 words) - 12:08, 3 June 2025
table is a set of elements unified by the atomic orbitals their valence electrons or vacancies lie in. The term seems to have been first used by Charles...
19 KB (2,487 words) - 22:13, 25 May 2025
the electron affinity of •CH3 was predicted to be negative). Such a species would decompose immediately by spontaneous ejection of an electron and would...
32 KB (3,479 words) - 12:55, 24 May 2025
another way, "high electron mobility"). Since GaAs has higher electron affinity, free electrons in the AlGaAs layer are transferred to the undoped GaAs layer...
21 KB (2,569 words) - 20:00, 23 May 2025
Negative methane (section Electron Affinity of Methane)
methane density conditions and, probably, a three body collision. The electron affinity (Eea) of an atom or molecule (A) is the energy difference between...
13 KB (1,413 words) - 00:08, 23 May 2025
determined by the electron affinity. This "vacuum level" does not correspond to any actual energy band and is poorly defined (electron affinity strictly speaking...
10 KB (1,365 words) - 00:19, 17 March 2025
group 13 or group 14, on the grounds of trends in ionisation energy, electron affinity, and electronegativity. Helium is an unreactive noble gas at standard...
24 KB (2,704 words) - 19:48, 4 June 2025
nonmetal) with greater electron affinity accepts one or more electrons to attain a stable electron configuration, and after accepting electrons an atom becomes...
18 KB (2,340 words) - 17:25, 5 February 2025
higher ionisation energies, higher electron affinities (nitrogen and the noble gases have negative electron affinities) and higher electronegativity values...
34 KB (4,571 words) - 09:31, 19 November 2024
extra electron to a hydrogen atom, called electron affinity of hydrogen. It is measured to be 0.754195(19) eV or 0.02771616(70) hartree (see Electron affinity...
6 KB (627 words) - 06:41, 25 May 2025
and oxides. Most are strongly paramagnetic because of their unpaired d electrons, as are many of their compounds. All of the elements that are ferromagnetic...
40 KB (4,500 words) - 18:28, 24 May 2025
difference of the metal-vacuum work function and the semiconductor-vacuum electron affinity. For an isolated metal, the work function Φ M {\displaystyle \Phi...
16 KB (2,095 words) - 19:42, 20 May 2025
Ionization energy (redirect from Electron binding energy)
example, the electron binding energy has the same magnitude as the electron affinity for the neutral chlorine atom. In another example, the electron binding...
51 KB (5,840 words) - 13:23, 28 May 2025
reduction potential in volts; electronegativity (revised Pauling); and electron affinity values (kJ/mol), for some metals and metalloids. The simplified entries...
30 KB (2,955 words) - 18:01, 30 May 2025