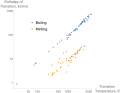
In thermodynamics, the enthalpy of fusion of a substance, also known as (latent) heat of fusion, is the change in its enthalpy resulting from providing...
12 KB (1,696 words) - 23:19, 4 May 2025
Melting (redirect from Fusion temperature)
entropy (S), known respectively as the enthalpy of fusion (or latent heat of fusion) and the entropy of fusion. Melting is therefore classified as a first-order...
10 KB (1,235 words) - 02:03, 25 March 2025
the enthalpy of vaporization (symbol ∆Hvap), also known as the (latent) heat of vaporization or heat of evaporation, is the amount of energy (enthalpy) that...
21 KB (1,061 words) - 03:48, 12 January 2025
Latent heat (redirect from Enthalpy of transformation)
Enthalpy of fusion Enthalpy of vaporization Ton of refrigeration – the power required to freeze or melt 2000 lb of water in 24 hours These “degrees of heat”...
21 KB (2,500 words) - 10:30, 18 May 2025
change. The enthalpy of sublimation (also called heat of sublimation) can be calculated by adding the enthalpy of fusion and the enthalpy of vaporization...
23 KB (2,429 words) - 02:37, 7 April 2025
Enthalpy (/ˈɛnθəlpi/ ) is the sum of a thermodynamic system's internal energy and the product of its pressure and volume. It is a state function in thermodynamics...
48 KB (6,109 words) - 22:51, 17 April 2025
Absolute zero (section Limit to the "degree of cold")
approach the change of state to liquid, and then to solid; and the sum of the enthalpy of vaporization (gas to liquid) and enthalpy of fusion (liquid to solid)...
41 KB (4,651 words) - 03:44, 15 May 2025
thermochemistry, the enthalpy of solution (heat of solution or enthalpy of solvation) is the enthalpy change associated with the dissolution of a substance in...
5 KB (681 words) - 06:11, 18 February 2025
Freezing (section Freezing of living organisms)
Helium-3 has a negative enthalpy of fusion at temperatures below 0.3 K. Helium-4 also has a very slightly negative enthalpy of fusion below 0.8 K. This means...
12 KB (1,405 words) - 16:52, 29 April 2025
} where Δ H fus {\displaystyle \Delta H_{\text{fus}}} is the enthalpy of fusion. Since this is a thermodynamic equation, the symbol T {\displaystyle...
2 KB (341 words) - 18:01, 3 January 2025
Carnot heat engine Conversion of scales of temperature Energy conversion efficiency Enthalpy Enthalpy of fusion Enthalpy of vaporization Entropy Equipartition...
105 KB (13,825 words) - 05:02, 16 May 2025
Section 6, Fluid Properties; Enthalpy of Fusion As quoted from various sources in: J.A. Dean (ed), Lange's Handbook of Chemistry (15th Edition), McGraw-Hill...
16 KB (187 words) - 16:47, 12 November 2023
Heat capacity (redirect from Specific heat of solids)
vaporization (see enthalpy of fusion and enthalpy of vaporization). Therefore, it should be considered a function C ( p , T ) {\displaystyle C(p,T)} of those two...
20 KB (2,906 words) - 18:44, 29 April 2025
Thermodynamic databases for pure substances (redirect from Enthalpy of transition)
designated ΔHtr. Enthalpy of fusion or melting. This applies to the transition of a solid to a liquid and is designated ΔHm. Enthalpy of vaporization. This...
26 KB (3,579 words) - 21:05, 9 May 2025
with the quantities: energy/mole of fuel energy/mass of fuel energy/volume of the fuel There are two kinds of enthalpy of combustion, called high(er) and...
27 KB (2,687 words) - 22:51, 21 April 2025
Melting point (redirect from Fusion point)
point together with its enthalpy of fusion. A basic melting point apparatus for the analysis of crystalline solids consists of an oil bath with a transparent...
27 KB (3,069 words) - 03:27, 30 March 2025
is the molar mass of the solvent. Tf is the freezing point of the pure solvent in kelvin. ΔHfus is the molar enthalpy of fusion of the solvent. The Kf...
2 KB (300 words) - 12:09, 19 March 2024
real gas. Physics portal Specific heat of melting (Enthalpy of fusion) Specific heat of vaporization (Enthalpy of vaporization) Frenkel line Heat capacity...
55 KB (8,537 words) - 22:17, 8 April 2025
Hilotherapy Phase-change material Enthalpy of fusion Singh, S P; Burgess, Singh (2008). "Performance Comparison of Thermal Insulated Packaging Boxes,...
6 KB (761 words) - 13:16, 14 April 2025
September 2017). Plasma Physics: An Introduction to Laboratory, Space, and Fusion Plasmas. Springer. ISBN 978-3-319-63427-2. M. Chaplin (20 August 2009)....
36 KB (4,388 words) - 17:18, 27 March 2025
per unit of propellant (either per unit of propellant mass, or per unit of propellant by Earth-weight) Specific melting heat, enthalpy of fusion; melting...
9 KB (1,163 words) - 16:09, 19 January 2025
account of it having a lower density than the other two isomers. Moreover, its enthalpy of fusion is lower than the enthalpies of fusion of both n-pentane...
11 KB (906 words) - 21:15, 16 May 2025
enthalpy of dissolution for the melting point depressant is often significantly greater (e.g. ΔH -57.61 kJ/mol for KOH) than the enthalpy of fusion for...
8 KB (840 words) - 11:17, 19 May 2024
Phase (matter) (redirect from Phases of matter)
enthalpy, associated with a solid to liquid transition is the enthalpy of fusion and that associated with a solid to gas transition is the enthalpy of...
13 KB (1,796 words) - 20:32, 4 March 2025
temperature. Most of the additional energy stored in the climate system since 1970 has accumulated in the oceans. The specific enthalpy of fusion (more commonly...
89 KB (9,586 words) - 12:04, 7 May 2025
P^{2}}}\right).} Apparent molar property Enthalpy change of solution Enthalpy of fusion Enthalpy of mixing Heat of dilution Ideal solution Lattice energy...
7 KB (1,298 words) - 12:22, 7 January 2024
Potassium carbonate (redirect from Salt of tartar)
production of soap and glass. Commonly, it can be found as the result of leakage of alkaline batteries. Potassium carbonate is a potassium salt of carbonic...
12 KB (943 words) - 03:31, 20 April 2025
Colligative properties (redirect from Colligative properties of solutions)
_{\mathrm {fus} }H} , where ΔfusH is the solvent molar enthalpy of fusion. The osmotic pressure of a solution is the difference in pressure between the...
13 KB (2,107 words) - 12:05, 14 May 2025
an example of Ostwald's step rule. The dynamically disordered nature of the β phase is partly responsible for the low enthalpy of fusion of silica. There...
13 KB (1,339 words) - 13:18, 25 April 2025
ideal gas enthalpy of formation, standard ideal gas Gibbs energy of formation, ideal gas heat capacity, enthalpy of vaporization, enthalpy of fusion, normal...
11 KB (1,414 words) - 10:13, 26 March 2025