Lithium hydride is an inorganic compound with the formula LiH. This alkali metal hydride is a colorless solid, although commercial samples are grey. Characteristic...
19 KB (1,922 words) - 10:36, 4 June 2025
Lithium aluminium hydride, commonly abbreviated to LAH, is an inorganic compound with the chemical formula Li[AlH4] or LiAlH4. It is a white solid, discovered...
35 KB (3,062 words) - 17:08, 29 May 2025
alkali metal hydrides react with metal halides. Lithium aluminium hydride (often abbreviated as LAH) arises from reactions of lithium hydride with aluminium...
21 KB (2,337 words) - 19:47, 20 April 2025
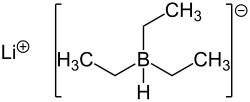
stronger reducing agent than lithium borohydride and lithium aluminium hydride. LiBHEt3 is prepared by the reaction of lithium hydride (LiH) and triethylborane...
8 KB (588 words) - 21:20, 23 May 2025
Diborane (redirect from Boron hydride)
sodium hydride (NaH), lithium hydride (LiH) or lithium aluminium hydride (LiAlH4): 8 BF3 + 6 LiH → B2H6 + 6 LiBF4 Lithium hydride used for this purpose must...
27 KB (2,667 words) - 05:23, 25 May 2025
explosives" along with lithium hydride (LiH) and lithium deuteride (LiD), beryllium (Be), uranium hydride (UH3), and plutonium hydride. Arms experts believe...
9 KB (984 words) - 18:06, 24 May 2025
in ethers, whilst remaining safer to handle than lithium aluminium hydride. Lithium borohydride may be prepared by the metathesis reaction, which occurs...
10 KB (833 words) - 02:07, 31 October 2023
synthesized in 1951 by treating dimethylberyllium, Be(CH3)2, with lithium aluminium hydride, LiAlH4. Purer BeH2 forms from the pyrolysis of di-tert-butylberyllium...
9 KB (781 words) - 23:33, 18 December 2024
generated in THF from lithium aluminium hydride. The reaction with lithium hydride in ether produces lithium aluminium hydride: AlH3 + LiH → Li[AlH4]...
29 KB (2,940 words) - 16:52, 28 May 2025
A nickel–metal hydride battery (NiMH or Ni–MH) is a type of rechargeable battery. The chemical reaction at the positive electrode is similar to that of...
37 KB (3,996 words) - 14:25, 18 May 2025
include lithium aluminium hydride (LiAlH4), lithium triethylborohydride, n-butyllithium and tert-butyllithium. Metallic lithium and its complex hydrides, such...
146 KB (14,240 words) - 04:46, 26 May 2025
N-Butyllithium (redirect from N-butyl lithium)
practice, they degrade upon aging, where a fine white precipitate (lithium hydride) is deposited and the color changes to orange. n-BuLi exists as a cluster...
18 KB (1,858 words) - 17:32, 24 May 2025
aluminium hydride. Lithium tetrahydridogallate was first reported by Finholt, Bond and Schlesinger. It is prepared by the reaction of lithium hydride and an...
6 KB (652 words) - 13:10, 28 May 2025
Mg(anthracene) + H2 → MgH2 the reaction of diethylmagnesium with lithium aluminium hydride product of complexed MgH2 e.g. MgH2.THF by the reaction of phenylsilane...
12 KB (1,102 words) - 04:09, 21 April 2025
Sodium hydride is the chemical compound with the empirical formula NaH. This alkali metal hydride is primarily used as a strong yet combustible base in...
14 KB (1,348 words) - 22:45, 25 May 2025
industrial process to produce ammonia since lithium hydride can be thermally decomposed back to lithium metal. Lithium nitride has been investigated as a storage...
9 KB (749 words) - 13:22, 28 March 2025
Binary compounds of hydrogen (redirect from Binary hydrides)
states. For example, monomeric lithium hydride has an enthalpy of formation of 139 kJ mol−1, whereas solid lithium hydride has an enthalpy of −91 kJ mol−1...
58 KB (3,932 words) - 01:17, 25 May 2025
suspension in diethyl ether, it reacts with lithium chloride to give the popular reagent lithium aluminium hydride: LiCl + NaAlH4 → LiAlH4 + NaCl The compound...
5 KB (456 words) - 10:28, 18 September 2024
Rubidium hydride is the hydride of rubidium. With the formula RbH, it is classified as an alkali metal hydride. It is a white solid and is insoluble in...
2 KB (77 words) - 19:28, 7 March 2025
the hydride anion was suggested by Gilbert N. Lewis in 1916 for group 1 and 2 salt-like compounds. In 1920, Moers electrolyzed molten lithium hydride (LiH)...
124 KB (12,916 words) - 13:28, 1 June 2025
problems. Lithium hydride was also in short supply, so Brown and Schlesinger needed to find a procedure that would allow them to use sodium hydride instead...
15 KB (1,391 words) - 19:14, 27 May 2025
Alkali metal (redirect from Lithium family)
between lithium and magnesium.: 76 The alkali metals also react similarly with hydrogen to form ionic alkali metal hydrides, where the hydride anion acts...
225 KB (24,165 words) - 11:13, 7 June 2025
Hydrogen compounds (section Hydrides)
the electrolysis of molten lithium hydride (LiH), producing a stoichiometric quantity of hydrogen at the anode. For hydrides other than group 1 and 2 metals...
14 KB (1,496 words) - 04:04, 4 April 2025
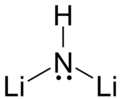
lithium amide and lithium hydride. LiNH2 + LiH → Li2NH + H2 The product is light-sensitive and can undergo disproportionation to lithium amide and characteristically...
3 KB (289 words) - 23:37, 2 February 2025
Hydrogen storage (section Metal hydrides)
hydrogen storage densities. Leading candidates are lithium hydride, sodium borohydride, lithium aluminium hydride and ammonia borane. A French company McPhy Energy...
135 KB (14,025 words) - 04:57, 28 May 2025
capture. In the case of lithium hydride, sodium hydride and potassium hydride molecules, this adduct decomposes and positronium hydride and the alkali positive...
9 KB (988 words) - 01:50, 28 May 2025
The lithium iron phosphate battery (LiFePO 4 battery) or LFP battery (lithium ferrophosphate) is a type of lithium-ion battery using lithium iron phosphate...
37 KB (3,503 words) - 13:20, 26 May 2025
aluminium hydride is lithium aluminium hydride (LiAlH4), which is used as a reducing agent in organic chemistry. It can be produced from lithium hydride and...
141 KB (15,298 words) - 14:15, 4 June 2025
shield meant melting lithium hydride and casting it into the form required. The form was a big truncated cone. Molten lithium hydride had to be poured into...
23 KB (2,744 words) - 12:13, 16 March 2025
A lithium-ion or Li-ion battery is a type of rechargeable battery that uses the reversible intercalation of Li+ ions into electronically conducting solids...
221 KB (23,718 words) - 12:10, 9 June 2025