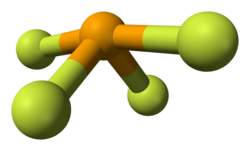
Selenium tetrafluoride (SeF4) is an inorganic compound. It is a colourless liquid that reacts readily with water. It can be used as a fluorinating reagent...
8 KB (534 words) - 13:27, 4 February 2025
explosive, and has been prepared directly from selenium tetrafluoride and trimethylsilyl azide. Selenium tetraazide is a yellow solid which precipitates...
2 KB (117 words) - 21:47, 13 December 2024
fluoride with tellurium or from the elements at 0 °C or by reacting selenium tetrafluoride with tellurium dioxide at 80 °C. Fluorine in nitrogen can react...
3 KB (243 words) - 06:48, 27 July 2022
tetrafluoride, PrF4 Protactinium tetrafluoride, PaF4 Radon tetrafluoride, RnF4 (predicted) Rhenium tetrafluoride, ReF4 Selenium tetrafluoride, SeF4, a liquid at standard...
4 KB (351 words) - 07:17, 9 September 2024
Sulfur tetrafluoride is a chemical compound with the formula SF4. It is a colorless corrosive gas that releases dangerous hydrogen fluoride gas upon exposure...
10 KB (837 words) - 22:43, 29 September 2024
(sulfur hexafluoride), selenium hexafluoride (SeF6) is more reactive and is a toxic pulmonary irritant. Selenium tetrafluoride is a laboratory-scale fluorinating...
96 KB (11,157 words) - 16:22, 15 May 2025
gas is produced along with the metal. Platinum tetrafluoride can form adducts with selenium tetrafluoride and bromine trifluoride. Volatile crystalline...
5 KB (435 words) - 23:14, 13 May 2025
reacts with selenium dioxide to form the Se(VI)-Se(IV) compound diselenium pentaoxide: SeO3 + SeO2 → Se2O5 It reacts with selenium tetrafluoride to form selenoyl...
7 KB (547 words) - 04:15, 17 April 2024
Selenium tetrabromide is an inorganic compound with a chemical formula SeBr4. Selenium tetrabromide could be produced by mixing elemental bromine and selenium:...
4 KB (264 words) - 19:45, 14 January 2024
both selenium tetrafluoride and tellurium tetrafluoride are known. Chalcogen chlorides and bromides have also been explored. In particular, selenium dichloride...
75 KB (8,309 words) - 14:58, 5 April 2025
Seleninyl fluoride (category Selenium(IV) compounds)
reaction of selenium tetrafluoride with water or selenium dioxide. SeF4 + H2O → SeOF2 + 2 HF SeF4 + SeO2 → 2 SeOF2 The reaction of selenium dioxide and...
4 KB (356 words) - 23:41, 10 December 2023
commonly available selenium chlorides, the other example being selenium monochloride, Se2Cl2. SeCl4 is used in the synthesis of other selenium compounds. The...
6 KB (434 words) - 05:50, 18 October 2024
Selenium fluoride may refer to: Selenium tetrafluoride (selenium(IV) fluoride), SeF4 Selenium hexafluoride (selenium(VI) fluoride), SeF6 Seleninyl fluoride...
304 bytes (61 words) - 03:02, 29 April 2025
concerns have led to interest in alternative fluorinating reagents. Selenium tetrafluoride, a liquid at room temperature, behaves similarly to SF4. Diethylaminosulfur...
6 KB (792 words) - 19:17, 7 February 2024
Selenium oxybromide – SeOBr2 Selenium oxydichloride – SeOCl2 Selenium tetrachloride – SeCl4 Selenium tetrafluoride – SeF4 Selenium trioxide – SeO3 Selenoyl...
121 KB (8,924 words) - 00:00, 18 May 2025
monosulfide 41831-80-5 F4Se selenium tetrafluoride 13465-66-2 F4Si silicon tetrafluoride 7783-61-1 F4Sn2 ditin tetrafluoride 130950-28-6 F4Ti titanium fluoride...
183 KB (107 words) - 23:35, 7 May 2025
an oxidation state of +2), sulfur, and selenium; tetrafluorides and hexafluorides exist for sulfur, selenium, and tellurium. The latter are stabilized...
158 KB (15,493 words) - 17:18, 29 April 2025
14457–70–6 SeCl4 selenium tetrachloride 10026–03–6 SeF2 selenium difluoride 70421–43–1 SeF4 selenium tetrafluoride 13465–66–2 SeF6 selenium hexafluoride 7783–79–1...
139 KB (120 words) - 13:10, 30 April 2025
Pentafluoroselenium hypofluorite (category Selenium(VI) compounds)
F5SeOC5F9. SeOF6 reacts with sulfur tetrafluoride to produce thionyl fluoride, thionyl tetrafluoride, sulfuryl fluoride, selenium hexafluoride and F5SOSeF5. SeOF6...
5 KB (374 words) - 23:21, 8 July 2024
example, it converts tungsten to tungsten hexafluoride and selenium to selenium tetrafluoride: W + 6 ClF → WF6 + 3 Cl2 Se + 4 ClF → SeF4 + 2 Cl2 FCl can...
5 KB (386 words) - 14:38, 14 April 2025
are somewhat similar: The tetrafluorides of S, Se, and Te hydrolyze and are Lewis acidic. Sulfur and selenium tetrafluorides are molecular while TeF4 is...
85 KB (8,520 words) - 05:00, 4 April 2025
with strong fluorinating agents such as bromine trifluoride and selenium tetrafluoride to form iodine pentafluoride. Iodyl fluoride can be reduced to elemental...
5 KB (363 words) - 07:36, 23 April 2025
carbonyl compounds. For example: COCl2 + 2 ROH → CO(OR)2 + 2 HCl Silicon tetrafluoride reacts with water to yield poorly-characterized oxyfluoride polymers...
17 KB (1,900 words) - 10:43, 5 May 2025
several new compounds, like bromine trifluoride, oxygen difluoride, selenium tetrafluoride and sulfur hexafluoride. In 1899 he was able to obtain pure beryllium...
2 KB (99 words) - 13:04, 7 October 2024
tetrabromide, PtBr4 Polonium tetrabromide, PoBr4 Protactinium tetrabromide, PaBr4 Selenium tetrabromide, SeBr4 Silicon tetrabromide, SiBr4 Tellurium tetrabromide...
1,004 bytes (106 words) - 20:07, 14 January 2024
groups. Sulfur reacts with fluorine to give the highly reactive sulfur tetrafluoride and the highly inert Sulfur hexafluoride. Whereas fluorine gives S(IV)...
11 KB (1,203 words) - 04:43, 23 February 2025
to decompose into polonium dichloride and excess chlorine, similar to selenium tetrachloride and tellurium tetrachloride. Polonium tetrachloride is either...
3 KB (202 words) - 22:51, 18 December 2022
prepared by refluxing palladium(II,IV) fluoride, PdII[PdIVF6], with selenium tetrafluoride, SeF4. Pd[PdF6] + SeF4 → 2PdF2 + SeF6 Like its lighter congener...
4 KB (190 words) - 16:40, 6 May 2025
Most difluorides and trifluorides have high boiling points, while most tetrafluorides and monofluorides fall in between. The term "fluoride volatility" is...
16 KB (1,116 words) - 03:10, 3 March 2025