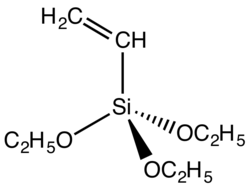
Silyl ethers are a group of chemical compounds which contain a silicon atom covalently bonded to an alkoxy group. The general structure is R1R2R3Si−O−R4...
10 KB (1,269 words) - 15:22, 16 March 2025
In organosilicon chemistry, silyl enol ethers are a class of organic compounds that share the common functional group R3Si−O−CR=CR2, composed of an enolate...
13 KB (1,369 words) - 22:56, 11 March 2025
Silylation (redirect from Silyl)
namely silyl ethers and silyl amines, are resilient toward basic conditions. Protection is typically done by reacting the functional group with a silyl halide...
10 KB (1,072 words) - 03:33, 15 March 2025
Organosilicon chemistry (redirect from Silyl halide)
extremely unstable. Silyl ethers have the connectivity Si-O-C. They are typically prepared by the reaction of alcohols with silyl chlorides: (CH 3) 3SiCl...
24 KB (2,354 words) - 22:08, 18 May 2025
alkali. The alkoxysilanes (silyl ethers) of the type R3Si(OR') are slow to hydrolyze. Compared to the silyl ethers, silyl acetates are faster to hydrolyze...
7 KB (814 words) - 18:16, 12 May 2025
addition to retaining all the known features that are associated with silyl ethers, such as their ease and selectivity of formation, their adaptability...
5 KB (582 words) - 23:47, 28 November 2023
direction, these silyl migrations produce silyl ethers as products which is driven by the stability of the oxygen-silicon bond. The silyl substituents can...
7 KB (986 words) - 03:19, 7 April 2024
Ge, Sn, Pb). Such compounds are considered ethers as well. Examples of such ethers are silyl enol ethers R3Si−O−CR=CR2 (containing the Si−O−C linkage)...
19 KB (1,841 words) - 14:31, 5 May 2025
(3-Aminopropyl)triethoxysilane (category Silyl ethers)
(3-Aminopropyl)triethoxysilane (APTES) is an aminosilane frequently used in the process of silanization, the functionalization of surfaces with alkoxysilane...
4 KB (317 words) - 21:00, 29 November 2023
Rubottom oxidation is a useful, high-yielding chemical reaction between silyl enol ethers and peroxyacids to give the corresponding α-hydroxy carbonyl product...
28 KB (3,396 words) - 17:19, 26 September 2024
a solid elastomer. Curing entails crosslinking by the hydrolysis of silyl ethers: 2 RSi(OCH3)2R' + H2O → [RSi(OCH3)R']2O + 2 CH3OH In a hydrolysis reaction...
2 KB (254 words) - 14:02, 30 January 2025
Tert-Butyldimethylsilyl chloride (redirect from Tert-Butyldimethylsilyl ethers)
tert-butyldimethylsilyl ethers: (Me3C)Me2SiCl + ROH → (Me3C)Me2SiOR + HCl These silyl ethers hydrolyze much more slowly than the trimethylsilyl ethers. It also can...
4 KB (276 words) - 22:02, 28 December 2024
Vinyltriethoxysilane (category Silyl ethers)
Vinyltriethoxysilane is an organosilicon compound with the formula (C2H5O)3SiCH=CH2. It is a colorless liquid. The compound is bifunctional, featuring...
3 KB (228 words) - 13:53, 19 September 2023
not isolated but trapped in situ with trimethylsilyl chloride to the silyl ether 9. In the next step, Gilman reagent 8 is a methylating reagent in nucleophilic...
9 KB (1,098 words) - 07:53, 11 June 2024
Polyester (section Silyl method)
chloride is converted with the trimethyl silyl ether of the alcohol component and production of trimethyl silyl chloride is obtained Aliphatic polyesters...
46 KB (5,396 words) - 23:52, 29 April 2025
the most industrially-important silicon α‑effect instead occurs with silyl ethers. Under hydrolysis condition, certain α-silane-terminated prepolymers...
11 KB (1,172 words) - 00:47, 19 April 2025
alkyl and vinyl silanes; aldehydes and ketones give silyl ethers, while esters provide alkyl silyl mixed acetals. Hydrosilylation has been called the "most...
8 KB (979 words) - 10:35, 27 April 2025
Triethoxysilane (category Silyl ethers)
bonds, triethoxysilane exhibits relatively low reactivity. Like most silyl ethers, triethoxysilane is susceptible to hydrolysis. As reducing agent, triethoxysilane...
2 KB (160 words) - 02:28, 26 August 2023
was converted to the silyl ether via asymmetric allylboration and silylation of the resulting alcohol. Ozonolysis of the silyl ether and Lindgren–Pinnick...
21 KB (2,166 words) - 04:03, 15 July 2024
preferred due to its lower cost. TMSI reacts with alkyl ethers (ROR′), forming silyl ethers (ROSiMe3) and iodoalkanes (RI) that can be hydrolyzed to...
5 KB (399 words) - 02:00, 21 January 2023
Methyltrimethoxysilane (category Silyl ethers)
Methyltrimethoxysilane is an organosilicon compound with the formula CH3Si(OCH3)3. It is a colorless, free-flowing liquid. It is a crosslinker in the preparation...
3 KB (235 words) - 15:05, 22 May 2024
unsaturases to give the vinyl ethers called plasmalogens: HOCH2CH(OH)CH2OC18H37 + [O] → HOCH2CH(OH)CH2OCH=CHC16H35 + H2O Silyl enol ether Jonathan Clayden; Greeves...
7 KB (807 words) - 11:17, 14 April 2025
Hexamethyldisiloxane (redirect from Bis(trimethylsilyl) ether)
2 HCl + O[Si(CH3)3]2 It also results from the hydrolysis of silyl ethers and other silyl-protected functional groups. HMDSO can be converted back to the...
6 KB (525 words) - 16:41, 19 April 2025
Reductions with hydrosilanes (section Ether cleavage)
hydrosilylation in the presence of hydrosilanes and fluoride. The resulting silyl ethers can be hydrolyzed with 1 M hydrochloric acid. Optimal yields of the hydrosilylation...
13 KB (1,457 words) - 10:07, 14 May 2025
-magnesium halide methylmagnesium chloride Alkylaluminium Al2R6 -aluminium trimethylaluminium Silyl ether R3SiOR -silyl ether trimethylsilyl triflate...
32 KB (1,230 words) - 13:26, 24 April 2025
used in organic synthesis as a reducing agent and as a precursor to silyl ethers. As one of the simplest trialkylsilanes that is a liquid at room temperature...
4 KB (323 words) - 03:56, 20 January 2025
protecting the acidic site of the reactant by turning it into an ether or a silyl ether to eliminate the labile proton from the solution prior to the Grignard...
12 KB (1,096 words) - 11:34, 5 April 2025
addition is an organic reaction and a type of aldol reaction between a silyl enol ether (R2C=CR−O−Si(CH3)3) and an aldehyde (R−CH=O) or formate (R−O−CH=O)...
9 KB (958 words) - 06:34, 11 February 2024
Danishefsky's diene (category Enol ethers)
in this aza Diels-Alder reaction: In the cycloaddition product, the silyl ether is a synthon for a carbonyl group through the enol. The methoxy group...
10 KB (963 words) - 14:09, 19 February 2025
to give a separable mixture of the starting lactone and the silyl ether. The silyl ether on hydrogenolysis followed by Collins oxidation gave the aldehyde...
33 KB (3,310 words) - 05:53, 6 February 2025