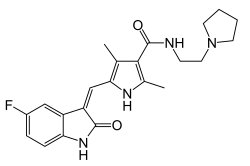
Sunitinib, sold under the brand name Sutent, is an anti-cancer medication. It is a small-molecule, multi-targeted receptor tyrosine kinase (RTK) inhibitor...
36 KB (3,366 words) - 03:36, 12 March 2025
randomized study of zanzalintinib in combination with nivolumab versus sunitinib in patients with advanced or metastatic non-clear cell RCC (nccRCC). Sharma...
5 KB (188 words) - 12:33, 5 September 2024
effectiveness of everolimus in carcinoid tumors have not been established. sunitinib (Sutent) is labeled for treatment of progressive, well-differentiated...
28 KB (2,471 words) - 03:07, 4 February 2025
(NSCLC). Sunitinib is an oral drug that inhibits the phosphorylation of all the VEGF receptors, PDGFR-ß, KIT FLT3, CSF1R and GDNF. Sunitinib is used in...
51 KB (4,735 words) - 08:15, 2 January 2024
approved within the past ten years. These treatments are: Nivolumab Axitinib Sunitinib Cabozantinib Everolimus Lenvatinib Pazopanib Bevacizumab Sorafenib Tivozanib...
100 KB (10,959 words) - 09:26, 11 February 2025
cytokine therapy (IL-2, interferon), kinase inhibitors (temsirolimus, sunitinib, sorafenib, pazopanib) and anti-angiogenic therapies (bevacizumab). "Clear...
3 KB (333 words) - 03:12, 23 February 2025
multiple-tyrosine-kinase inhibitor sunitinib (marketed as Sutent) can be considered.: 26 and 31 The effectiveness of imatinib and sunitinib depends on the genotype...
46 KB (4,782 words) - 13:48, 30 April 2025
inhibitor that is effective both as a senolytic and as therapy for CML. Sunitinib, an inhibitor of the receptors for FGF, PDGF and VEGF is also based on...
10 KB (1,179 words) - 18:31, 8 August 2024
shut down in 2003, after developing the pioneering kinase inhibitor drug sunitinib (Sutent). Sugen was founded in 1991 in Redwood City, California, by veteran...
8 KB (823 words) - 17:00, 26 September 2024
small molecules that inhibit the tyrosine kinases stimulated by VEGF: sunitinib, sorafenib, axitinib, and pazopanib (some of these therapies target VEGF...
14 KB (1,693 words) - 18:18, 18 October 2024
phosphate. It was developed by SUGEN as SU11654, a sister compound to sunitinib, which was later approved for human therapies. Toceranib is a tyrosine...
7 KB (368 words) - 19:18, 1 May 2025
MedlinePlus. Clinical trial number NCT01835158 for "Cabozantinib-s-malate or Sunitinib Malate in Treating Patients With Previously Untreated Locally Advanced...
24 KB (2,062 words) - 19:34, 28 March 2025
trial number NCT02231749 for "Nivolumab Combined With Ipilimumab Versus Sunitinib in Previously Untreated Advanced or Metastatic Renal Cell Carcinoma (CheckMate...
47 KB (4,859 words) - 18:32, 1 May 2025
removal and is causing symptoms, targeted therapy with everolimus or sunitinib can reduce symptoms and slow progression of the disease. Standard cytotoxic...
129 KB (13,579 words) - 11:28, 3 May 2025
L01EN02 Pemigatinib L01EN03 Infigratinib L01EN04 Futibatinib L01EX01 Sunitinib L01EX02 Sorafenib L01EX03 Pazopanib L01EX04 Vandetanib L01EX05 Regorafenib...
13 KB (932 words) - 04:36, 23 December 2024
jobs, and several programs were transferred to Pfizer. These included sunitinib (Sutent), a cancer medication which was approved for human use by the...
194 KB (17,498 words) - 19:55, 13 April 2025
treatment for patients with advanced kidney cancer after failure of either sunitinib or sorafenib" (Press release). Novartis. 30 March 2009. Archived from...
27 KB (2,507 words) - 13:06, 13 April 2025
stabilizers, amiodarone, interferon alpha, tyrosine kinase inhibitors such as sunitinib Central hypothyroidism Lesions compressing the pituitary (pituitary adenoma...
75 KB (7,734 words) - 10:44, 17 April 2025
Masitinib Motesanib Nintedanib Pazopanib Radotinib Quizartinib Ripretinib Sunitinib Sorafenib Toceranib Antibodies: Olaratumab Ramucirumab Tovetumab RET (GFL)...
93 KB (8,717 words) - 03:14, 10 March 2025
Retrieved 20 July 2023. "A Study of Abemaciclib in Combination With Sunitinib in Metastatic Renal Cell Carcinoma". www.clinicaltrials.gov. Retrieved...
18 KB (1,467 words) - 15:22, 19 January 2025
phase 3 study (Checkmate 9ER) that showed this regimen to be superior to sunitinib, which led to its FDA approval in January 2021. He also co-led with Robert...
19 KB (1,694 words) - 21:01, 16 April 2025
reaction of esters with hydrazine: An applied example is a synthesis of sunitinib begins by mixing 5-fluoroisatin slowly into hydrazine hydrate. After 4...
5 KB (446 words) - 03:46, 9 January 2025
patients and as an anti-fatigue agent in kidney cancer patients treated with sunitinib. There is a single case report of its use in the successful treatment...
5 KB (340 words) - 20:58, 11 February 2025
contributed to early and late stage clinical trials of temozolomide, sunitinib, everolimus, and peptide receptor radiotherapy for neuroendocrine tumors...
9 KB (760 words) - 23:01, 26 September 2023
vinblastine, vincristine, vindesine, imatinib, irinotecan, sorafenib, sunitinib, vemurafenib, temsirolimus, anastrozole, gefitinib; azole antifungals:...
81 KB (7,834 words) - 19:44, 15 April 2025
Masitinib Motesanib Nintedanib Pazopanib Radotinib Quizartinib Ripretinib Sunitinib Sorafenib Toceranib Antibodies: Olaratumab Ramucirumab Tovetumab RET (GFL)...
12 KB (966 words) - 04:46, 15 July 2024
Masitinib Motesanib Nintedanib Pazopanib Radotinib Quizartinib Ripretinib Sunitinib Sorafenib Toceranib Antibodies: Olaratumab Ramucirumab Tovetumab RET (GFL)...
150 KB (15,796 words) - 01:16, 20 April 2025
This inhibitor is a highly selective Bcr-Abl tyrosine kinase inhibitor. Sunitinib is an oral tyrosine kinase inhibitor that acts upon vascular endothelial...
44 KB (5,283 words) - 12:50, 27 December 2024
the availability of tyrosine kinase inhibitors, such as imatinib and sunitinib that can block the activity of c-KIT pharmacologically. It is expected...
79 KB (7,943 words) - 20:24, 1 May 2025
Masitinib Motesanib Nintedanib Pazopanib Radotinib Quizartinib Ripretinib Sunitinib Sorafenib Toceranib Antibodies: Olaratumab Ramucirumab Tovetumab RET (GFL)...
32 KB (3,436 words) - 04:03, 2 May 2025