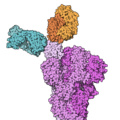
Casirivimab/imdevimab, sold under the brand name REGEN‑COV among others, is a combination medicine used for the treatment and prevention of COVID‑19....
50 KB (3,514 words) - 21:45, 11 July 2025
public domain. "Casirivimab injection, solution, concentrate Imdevimab injection, solution, concentrate REGEN-COV – casirivimab and imdevimab kit". DailyMed...
90 KB (13,312 words) - 21:46, 26 July 2025
Carvykti carzelesin (INN) carzenide (INN) Casgevy casimersen casirivimab and imdevimab Casodex casokefamide (INN) casopitant (USAN, INN) caspofungin...
16 KB (757 words) - 05:06, 19 June 2024
the monoclonal antibody therapies bamlanivimab/etesevimab and casirivimab/imdevimab were given emergency use authorizations by the US Food and Drug...
49 KB (5,031 words) - 08:23, 18 July 2025
October 2020. "Casirivimab injection, solution, concentrate Imdevimab injection, solution, concentrate REGEN-COV- casirivimab and imdevimab kit". DailyMed...
75 KB (7,325 words) - 15:37, 23 July 2025
November 2021. "Japan becomes first country to approve Ronapreve (casirivimab and imdevimab) for the treatment of mild to moderate COVID-19". Roche (Press...
146 KB (4,507 words) - 05:10, 18 July 2025
J06BD04 Ansuvimab J06BD05 Sotrovimab J06BD06 Regdanvimab J06BD07 Casirivimab and imdevimab J06BD08 Nirsevimab J06BD09 Sipavibart Immune sera and immunoglobulins...
2 KB (263 words) - 04:26, 7 March 2025
rolling reviews of data on the use of the monoclonal antibodies casirivimab/imdevimab, bamlanivimab/etesevimab, and bamlanivimab for the treatment of...
19 KB (1,631 words) - 20:05, 29 May 2025
the monoclonal antibody therapies bamlanivimab/etesevimab and casirivimab/imdevimab were found to reduce the number of hospitalizations, emergency room...
70 KB (6,626 words) - 21:20, 18 July 2025
of plasma in COVID-19 in December 2021. Monoclonal antibodies (casirivimab/imdevimab) were developed for the treatment of COVID-19. On June 7, 2021,...
21 KB (2,430 words) - 00:06, 19 July 2025
treatments – bamlanivimab/etesevimab (administered together) and casirivimab/imdevimab – to limit their use to only when the recipients are likely to have...
18 KB (1,341 words) - 20:05, 29 May 2025
Bebtelovimab§ Casirivimab† (+imdevimab) Cilgavimab† (+tixagevimab)† Clesrovimab Diridavumab§ Etesevimab† Exbivirumab§ Foravirumab§ Imdevimab† (+casirivimab) Libivirumab§...
25 KB (2,697 words) - 18:32, 22 July 2025
clinic at AdventHealth Orlando, COVID-19 patients were treated with Casirivimab/imdevimab and Bamlanivimab to keep them out of the hospital. In March 2022...
38 KB (3,044 words) - 19:24, 7 July 2025
clinic at AdventHealth Orlando, COVID-19 patients were treated with Casirivimab/imdevimab and Bamlanivimab to keep them out of the hospital. On January 4...
202 KB (16,608 words) - 21:36, 22 July 2025
antibodies Bamlanivimab/etesevimab Bamlanivimab Etesevimab Bebtelovimab Casirivimab/imdevimab Regdanvimab Sarilumab Sotrovimab Tixagevimab/cilgavimab Tocilizumab...
182 KB (23,962 words) - 23:19, 12 February 2025
against Delta on its own. At high enough concentrations, casirivimab, etesevimab and imdevimab appear to still be effective. A preprint study suggests...
129 KB (12,263 words) - 02:06, 13 July 2025