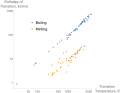
Enthalpy (/ˈɛnθəlpi/ ) is the sum of a thermodynamic system's internal energy and the product of its pressure and volume. It is a state function in thermodynamics...
48 KB (6,108 words) - 20:45, 18 July 2025
the enthalpy of vaporization (symbol ∆Hvap), also known as the (latent) heat of vaporization or heat of evaporation, is the amount of energy (enthalpy) that...
21 KB (1,061 words) - 03:48, 12 January 2025
thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of enthalpy during the formation of 1 mole...
29 KB (1,879 words) - 21:49, 30 April 2025
In thermodynamics, the enthalpy of fusion of a substance, also known as (latent) heat of fusion, is the change in its enthalpy resulting from providing...
12 KB (1,696 words) - 23:19, 4 May 2025
Heat of combustion (redirect from Standard enthalpy change of combustion)
fuel energy/mass of fuel energy/volume of the fuel There are two kinds of enthalpy of combustion, called high(er) and low(er) heat(ing) value, depending on...
27 KB (2,687 words) - 19:34, 24 May 2025
Bond-dissociation energy (redirect from Bond dissociation enthalpy)
the standard enthalpy change when A−B is cleaved by homolysis to give fragments A and B, which are usually radical species. The enthalpy change is temperature-dependent...
24 KB (2,441 words) - 16:09, 24 May 2025
In thermodynamics, the enthalpy of mixing (also heat of mixing and excess enthalpy) is the enthalpy liberated or absorbed from a substance upon mixing...
12 KB (1,889 words) - 10:40, 3 July 2025
The standard enthalpy of reaction (denoted Δ H reaction ⊖ {\displaystyle \Delta H_{\text{reaction}}^{\ominus }} ) for a chemical reaction is the difference...
16 KB (2,425 words) - 08:47, 25 November 2024
In thermodynamics, the enthalpy of sublimation, or heat of sublimation, is the heat required to sublimate (change from solid to gas) one mole of a substance...
3 KB (180 words) - 05:24, 26 May 2025
In thermochemistry, the enthalpy of solution (heat of solution or enthalpy of solvation) is the enthalpy change associated with the dissolution of a substance...
5 KB (681 words) - 06:11, 18 February 2025
stagnation enthalpy of a fluid is the static enthalpy of the fluid at a stagnation point. The stagnation enthalpy is also called total enthalpy. At a point...
4 KB (634 words) - 18:34, 18 July 2025
Hess's law (section Use of enthalpies of formation)
and physician who published it in 1840. The law states that the total enthalpy change during the complete course of a chemical reaction is independent...
11 KB (1,424 words) - 12:46, 16 June 2025
In chemistry and thermodynamics, the enthalpy of neutralization (ΔnH) is the change in enthalpy that occurs when one equivalent of an acid and a base undergo...
3 KB (362 words) - 13:59, 22 April 2025
An enthalpy–entropy chart, also known as the H–S chart or Mollier diagram, plots the total heat against entropy, describing the enthalpy of a thermodynamic...
7 KB (804 words) - 13:54, 9 August 2024
an exothermic reaction is a "reaction for which the overall standard enthalpy change ΔH⚬ is negative." Exothermic reactions usually release heat. The...
5 KB (552 words) - 07:35, 19 March 2024
chemical reaction to the change in temperature, T, given the standard enthalpy change, ΔrH⊖, for the process. The subscript r {\displaystyle r} means...
22 KB (3,079 words) - 05:42, 30 June 2025
In chemistry, the enthalpy of atomization (also atomisation in British English) is the enthalpy change that accompanies the total separation of all atoms...
2 KB (259 words) - 05:28, 26 July 2025
lattice energy (or more precisely enthalpy), which cannot otherwise be measured directly. The lattice enthalpy is the enthalpy change involved in the formation...
7 KB (941 words) - 22:38, 25 March 2025
In thermodynamics, enthalpy–entropy compensation is a specific example of the compensation effect. The compensation effect refers to the behavior of a...
19 KB (2,793 words) - 16:36, 28 May 2025
Gibbs free energy (redirect from Free enthalpy)
{\textstyle U} is the internal energy of the system H {\textstyle H} is the enthalpy of the system S {\textstyle S} is the entropy of the system T {\textstyle...
33 KB (4,550 words) - 21:59, 19 June 2025
performance. Since it is a constant-enthalpy process, it can be used to experimentally measure the lines of constant enthalpy (isenthalps) on the ( p , T )...
33 KB (4,461 words) - 19:50, 21 April 2025
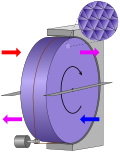
Thermal wheel (redirect from Enthalpy wheel)
thermal wheel, also known as a rotary heat exchanger, or rotary air-to-air enthalpy wheel, energy recovery wheel, or heat recovery wheel, is a type of energy...
15 KB (2,091 words) - 08:33, 2 May 2025
Ionization energy (redirect from Ionisation enthalpy)
giving two radical fragments A and B and subsequent evaluation of the enthalpy change Bond energy, the average measure of a chemical bond's strength,...
51 KB (5,844 words) - 16:50, 28 June 2025
Latent heat (redirect from Enthalpy of transformation)
as the liquid's sensible heat onto the surface. The large value of the enthalpy of condensation of water vapor is the reason that steam is a far more effective...
21 KB (2,500 words) - 12:04, 29 July 2025
thermodynamics, it is a thermodynamic process with an increase in the enthalpy H (or internal energy U) of the system. In an endothermic process, the...
9 KB (941 words) - 16:28, 17 July 2025
chemical bond. It is sometimes called the mean bond, bond enthalpy, average bond enthalpy, or bond strength. IUPAC defines bond energy as the average...
10 KB (1,317 words) - 12:02, 25 May 2025
Wind tunnel (redirect from High enthalpy wind tunnel)
transonic wind tunnel Supersonic wind tunnel Hypersonic wind tunnel High enthalpy wind tunnel Wind tunnels are also classified by the orientation of air...
44 KB (5,581 words) - 01:16, 29 July 2025
Lattice energy (redirect from Lattice enthalpy)
and so the value of the lattice enthalpy is also negative (and exothermic). Both, lattice energy and lattice enthalpy are identical at 0 K and the difference...
14 KB (1,828 words) - 05:31, 26 July 2025
ratio of static enthalpy change in the rotor to the static enthalpy change in the stage. Various definitions exist in terms of enthalpies, pressures or...
10 KB (1,558 words) - 20:51, 22 June 2025
Hydration energy (redirect from Hydration enthalpy)
In chemistry, hydration energy (also hydration enthalpy) is the amount of energy released when one mole of ions undergoes solvation. Hydration energy is...
3 KB (342 words) - 19:54, 15 February 2025