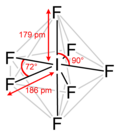
In chemistry, a hypervalent molecule (the phenomenon is sometimes colloquially known as expanded octet) is a molecule that contains one or more main group...
39 KB (4,517 words) - 13:25, 25 April 2025
definitively excluding the role of d-orbital hybridisation in bonding in hypervalent compounds of second-row (period 3) elements, ending a point of contention...
33 KB (3,204 words) - 20:53, 19 May 2025
Octet rule (section Hypervalent molecules)
attain this configuration in compounds. There are, however, some hypervalent molecules in which the 3d level may play a part in the bonding, although this...
23 KB (2,861 words) - 21:23, 30 May 2025
Sulfur hexafluoride (category Hypervalent molecules)
of six fluorine atoms attached to a central sulfur atom. It is a hypervalent molecule.[citation needed] Typical for a nonpolar gas, SF 6 is poorly soluble...
43 KB (4,163 words) - 19:24, 27 May 2025
Three-center four-electron bond (section Examples of molecules exhibiting three-center four-electron bonding)
4-electron (3c–4e) bond is a model used to explain bonding in certain hypervalent molecules such as tetratomic and hexatomic interhalogen compounds, sulfur...
11 KB (1,213 words) - 17:27, 4 April 2025
Sulfur dioxide (category Hypervalent molecules)
known to medieval alchemists as "volatile spirit of sulfur". SO2 is a bent molecule with C2v symmetry point group. A valence bond theory approach considering...
62 KB (7,592 words) - 22:41, 22 May 2025
Resonance (chemistry) (section Aromatic molecules)
participation of d orbitals is unimportant, and the bonding of so-called hypervalent molecules are, for the most part, better explained by charge-separated contributing...
42 KB (5,100 words) - 13:19, 23 May 2025
Sulfur trioxide (category Hypervalent molecules)
violently with water to produce highly corrosive sulfuric acid. Hypervalent molecule Sulfur trioxide pyridine complex Greenwood, Norman N.; Earnshaw,...
19 KB (1,668 words) - 01:16, 26 May 2025
Phosphorus pentachloride (category Hypervalent molecules)
Gaseous and molten PCl5 is a neutral molecule with trigonal bipyramidal geometry and (D3h) symmetry. The hypervalent nature of this species (as well as...
18 KB (1,565 words) - 12:22, 24 May 2025
Triiodide (category Hypervalent molecules)
iodine-atom. In the molecular orbital model, a common explanation for the hypervalent bonding on the central iodine involves a three-center four-electron bond...
16 KB (1,587 words) - 12:35, 30 May 2025
These are the hypervalent organoiodines, often called iodanes after the IUPAC rule used to name them. These iodine compounds are hypervalent because the...
18 KB (1,940 words) - 22:01, 27 March 2025
also be moved in the same way to create resonance structures for hypervalent molecules such as sulfur hexafluoride, which is the correct description according...
16 KB (2,159 words) - 05:20, 16 May 2025
VALBOND (section Non-hypervalent molecules)
used by many force fields, and allows the VALBOND method to handle hypervalent molecules and transition metal complexes. The VALBOND energy term has been...
7 KB (1,245 words) - 02:04, 27 May 2025
Sulfur tetrachloride (category Hypervalent molecules)
noncoordinating anions. In contrast to this tetrachloride, SF4 is a neutral molecule. It decomposes above −30 °C (242 K) to sulfur dichloride and chlorine....
3 KB (282 words) - 20:46, 3 September 2024
Difluorodisulfanedifluoride (category Hypervalent molecules)
fluorine atoms as Shyp (for hypervalent) and Stop. The fluorine atoms are labelled Ftop attached to Stop, and on the hypervalent S atom: Fcis, the closest...
11 KB (1,065 words) - 00:47, 13 December 2024
Martin's sulfurane (category Hypervalent molecules)
solid that easily undergoes sublimation. The compound is an example of a hypervalent sulfur compound called a sulfurane. As such, the sulfur adopts a see-saw...
3 KB (165 words) - 12:24, 6 April 2023
in the periodic table.) Lower-period elements, however, may form hypervalent molecules, such as phosphorus pentafluoride or sulfur hexafluoride. The reactivity...
85 KB (8,520 words) - 07:06, 25 May 2025
than predicted by the octet rule, as explained in the article on hypervalent molecules. The mechanisms of their reactions differ from organic compounds...
30 KB (3,224 words) - 15:33, 23 May 2025
electronegativity of the two bonded atoms. Pauling also considered hypervalent molecules, in which main-group elements have apparent valences greater than...
40 KB (2,914 words) - 12:59, 11 January 2025
Tetraethylammonium trichloride (category Hypervalent molecules)
Tetraethylammonium trichloride (also known as Mioskowski reagent) is a chemical compound with the formula [NEt4][Cl3] consisting of a tetraethylammonium...
7 KB (612 words) - 04:31, 21 February 2023
Iodine heptafluoride (category Hypervalent molecules)
bipyramidal structure, with D5h symmetry, as predicted by VSEPR theory. The molecule can undergo a pseudorotational rearrangement called the Bartell mechanism...
8 KB (728 words) - 16:14, 9 February 2025
Sulfur tetrafluoride (category Hypervalent molecules)
3 pm and S–Feq = 154.2 pm. It is typical for the axial ligands in hypervalent molecules to be bonded less strongly. The 19F NMR spectrum of SF4 reveals...
10 KB (837 words) - 22:43, 29 September 2024
rearrangement 2,3-Wittig rearrangement Directed ortho metalation Ate complex Hypervalent molecule Potassium tetraphenylborate Awards Otto Hahn Prize for Chemistry...
7 KB (730 words) - 15:41, 30 May 2025
Fluorine (section Discrete molecules)
Noury, S.; Silvi, B.; Gillespie, R. J. (2002). "Chemical Bonding in Hypervalent Molecules: Is the Octet Rule Relevant?" (PDF). Inorganic Chemistry. 41 (8):...
158 KB (15,507 words) - 15:46, 29 May 2025
fundamental contributions in polymer science. Beta-lactam Carbene Hypervalent molecule Polyoxymethylene Pyrethrin Triphenylphosphine phenylimide Heidegger...
17 KB (1,551 words) - 23:52, 26 May 2025
Linnett double-quartet theory (category Molecules)
the phosphorus atom. Thus, the molecule is assumed to expand its bonding beyond the octet, a situation known as hypervalent bonding. LDQ theory, however...
70 KB (7,777 words) - 13:05, 22 September 2024
Pentamethylbismuth (category Hypervalent molecules)
bound to a bismuth atom with formula Bi(CH3)5. It is an example of a hypervalent compound. The molecular shape is trigonal bipyramid. Pentamethylbismuth...
5 KB (454 words) - 17:34, 25 July 2024
Disulfur decafluoride (category Hypervalent molecules)
discovered in 1934 by Denbigh and Whytlaw-Gray. Each sulfur atom of the S2F10 molecule is octahedral, and surrounded by five fluorine atoms and one sulfur atom...
8 KB (654 words) - 01:34, 10 May 2024
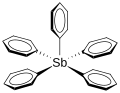
Pentaphenylantimony (category Hypervalent molecules)
of several studies, and a definite ground state remains uncertain. The molecule adopts a roughly square pyramidal shape in the unsolvated crystal. In crystals...
6 KB (537 words) - 01:25, 22 December 2024
SeCl4 has often been used as an example for teaching VSEPR rules of hypervalent molecules. As such, one would predict four bonds but five electron groups...
6 KB (434 words) - 05:50, 18 October 2024