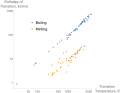
Thermodynamic databases contain information about thermodynamic properties for substances, the most important being enthalpy, entropy, and Gibbs free...
26 KB (3,579 words) - 21:05, 9 May 2025
a thermodynamic property. Conjugate variables Dimensionless numbers Intensive and extensive properties Thermodynamic databases for pure substances Thermodynamic...
7 KB (334 words) - 05:35, 12 April 2022
Chemical equilibrium (section Pure substances)
concentrations.) (See Thermodynamic databases for pure substances.) Note that the second equation is just the initial constraints for minimization. This...
44 KB (6,727 words) - 15:12, 28 July 2025
Heat capacity (category Thermodynamic properties)
Thermodynamic equations – Equations in thermodynamics Thermodynamic databases for pure substances – Thermodynamic properties list Heat equation – Partial differential...
20 KB (2,908 words) - 18:59, 22 June 2025
{1}{T_{\text{fus}}}}\right)} Enthalpy of vaporization Heat capacity Thermodynamic databases for pure substances Joback method (Estimation of the heat of fusion from...
12 KB (1,696 words) - 23:19, 4 May 2025
spectroscopy Principle of maximum work Reaction Calorimeter Thermodynamic databases for pure substances Thermodynamics Thomsen-Berthelot principle Julius Thomsen...
7 KB (788 words) - 18:00, 26 June 2025
covariance flux (eddy correlation, eddy flux) Enthalpy Thermodynamic databases for pure substances Partington, J.R. (1949). An Advanced Treatise on Physical...
4 KB (524 words) - 18:06, 8 July 2025
Thermal expansion (section For various materials)
Heat capacity – Physical property of matter Thermodynamic databases for pure substances – Thermodynamic properties list Material properties (thermodynamics)...
49 KB (6,124 words) - 22:14, 2 July 2025
Chemical thermodynamics (redirect from Chemical thermodynamic)
"chemical work", a misnomer for the free energy of another chemical process. Thermodynamic databases for pure substances laws of thermodynamics Ott, Bevan...
22 KB (3,079 words) - 16:41, 6 June 2025
irreversible. The thermodynamic concept was referred to by Scottish scientist and engineer William Rankine in 1850 with the names thermodynamic function and...
111 KB (14,228 words) - 03:00, 30 June 2025
value depends on customary choices of standard state for the species. The activity of pure substances in condensed phases (solids and liquids) is taken as...
22 KB (3,015 words) - 03:34, 26 May 2025
with respect to that substance is possible. By suitable thermodynamic operations, the pure substance reservoir can be dealt with as a closed system. Its internal...
30 KB (4,100 words) - 08:39, 22 July 2025
the desired temperature has been reached: (see also Thermodynamic databases for pure substances) Constant-pressure calorimeter, enthalpy-meter, active...
43 KB (3,620 words) - 22:17, 23 June 2025
Specific heat capacity (category Thermodynamic properties)
Table of specific heat capacities Thermal mass Thermodynamic databases for pure substances Thermodynamic equations Volumetric heat capacity IUPAC, Compendium...
55 KB (8,515 words) - 12:09, 29 July 2025
Chemical potential (category Thermodynamic properties)
1873 paper A Method of Geometrical Representation of the Thermodynamic Properties of Substances by Means of Surfaces, Gibbs introduced the preliminary outline...
33 KB (4,561 words) - 03:57, 24 June 2025
In thermodynamics, the thermodynamic free energy is one of the state functions of a thermodynamic system. The change in the free energy is the maximum...
28 KB (4,048 words) - 15:22, 26 May 2025
Entropy (classical thermodynamics) (redirect from Thermodynamic entropy)
entropy (from Greek τρoπή (tropḗ) 'transformation') is a property of a thermodynamic system that expresses the direction or outcome of spontaneous changes...
17 KB (2,587 words) - 15:45, 28 December 2024
CALPHAD (category Thermodynamic free energy)
mixing Miedema's Model Computational thermodynamics Thermodynamic_databases_for_pure_substances Kaufman L.; Bernstein H. (1970). Computer Calculation...
8 KB (968 words) - 13:39, 30 September 2024
Phase diagram (redirect from Thermodynamic chart)
chart used to show conditions (pressure, temperature, etc.) at which thermodynamically distinct phases (such as solid, liquid or gaseous states) occur and...
22 KB (2,528 words) - 18:18, 29 July 2025
Thermodynamic temperature, also known as absolute temperature, is a physical quantity that measures temperature starting from absolute zero, the point...
101 KB (13,298 words) - 18:19, 24 July 2025
chemical substances. In thermodynamic open systems, mass (of substances) may flow in and out of the system boundaries. The first law of thermodynamics for open...
48 KB (6,108 words) - 20:45, 18 July 2025
Chemical element (redirect from Pure element)
Mixtures are materials containing different chemical substances; that means (in case of molecular substances) that they contain different types of molecules...
76 KB (10,679 words) - 20:00, 20 July 2025
In thermodynamics, heat is energy in transfer between a thermodynamic system and its surroundings by such mechanisms as thermal conduction, electromagnetic...
92 KB (12,982 words) - 12:04, 29 July 2025
Helmholtz free energy (category Thermodynamic free energy)
for a pure substance (together with its partial derivatives) can be used to determine all other thermodynamic properties for the substance. See, for example...
23 KB (4,205 words) - 10:36, 5 August 2025
Properties of water (redirect from Water substance)
Also, fairly pure silicon has a negative coefficient of thermal expansion for temperatures between about 18 and 120 kelvins. Other substances that expand...
90 KB (9,586 words) - 07:11, 31 July 2025
melt can co-exist with crystals in thermodynamic equilibrium. Liquidus and solidus are mostly used for impure substances (mixtures) such as glasses, metal...
10 KB (1,149 words) - 20:51, 11 July 2025
thermodynamics establishes the concept of entropy as a physical property of a thermodynamic system. It predicts whether processes are forbidden despite obeying...
108 KB (15,568 words) - 04:28, 26 July 2025
Molar heat capacity (category Thermodynamic properties)
Heat capacity ratio Statistical mechanics Thermodynamic equations Thermodynamic databases for pure substances Heat equation Heat transfer coefficient Heat...
50 KB (5,707 words) - 12:54, 15 July 2025
Freezing-point depression (category Amount of substance)
freezing point than the pure solvent or solid because the chemical potential of the solvent in the mixture is lower than that of the pure solvent, the difference...
19 KB (2,362 words) - 09:08, 17 July 2025
Viscosity (section Selected substances)
in the sense that predictions for viscosity can be obtained using parameters fitted to other, pure-fluid thermodynamic properties or transport properties...
100 KB (11,467 words) - 19:48, 24 May 2025