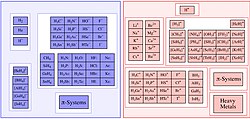
A conjugate acid, within the Brønsted–Lowry acid–base theory, is a chemical compound formed when an acid gives a proton (H+) to a base—in other words,...
13 KB (1,310 words) - 14:02, 17 May 2025
of this theory is that when an acid and a base react with each other, the acid forms its conjugate base, and the base forms its conjugate acid by exchange...
16 KB (1,949 words) - 16:30, 29 May 2025
are called the acid–base theories, for example, Brønsted–Lowry acid–base theory. Their importance becomes apparent in analyzing acid–base reactions for...
36 KB (4,977 words) - 16:16, 16 June 2025
accept a pair of electrons. The conjugate base of a Brønsted–Lowry acid is also a Lewis base as loss of H+ from the acid leaves those electrons which were...
22 KB (2,771 words) - 02:54, 24 June 2025
Conjugation (redirect from Conjugate)
bacteria Conjugate vaccine, in immunology Conjugation (biochemistry), covalently linking a biomolecule with another molecule Conjugate (acid-base theory), a...
3 KB (407 words) - 14:29, 14 December 2024
conjugate base. This reaction is referred to as protolysis. The protonated form (HA) of an acid is also sometimes referred to as the free acid. Acid–base...
47 KB (5,960 words) - 15:34, 29 May 2025
In acid catalysis and base catalysis, a chemical reaction is catalyzed by an acid or a base. By Brønsted–Lowry acid–base theory, the acid is the proton...
7 KB (888 words) - 08:18, 24 May 2025
An acid–base titration is a method of quantitative analysis for determining the concentration of Brønsted-Lowry acid or base (titrate) by neutralizing...
24 KB (2,650 words) - 21:07, 28 June 2025
the context of acid–base reactions. The chemical species HA is an acid that dissociates into A−, called the conjugate base of the acid, and a hydrogen...
103 KB (11,534 words) - 07:28, 2 June 2025
represents the general reaction between a base (B) and water to produce a conjugate acid (BH+) and a conjugate base (OH−): B ( aq ) + H 2 O ( l ) ↽ − − ⇀...
26 KB (3,035 words) - 02:15, 29 June 2025
PH indicator (redirect from Acid-base indicator)
and "Ind+" for the conjugate acid of the indicator. The ratio of concentration of conjugate acid/base to concentration of the acidic/basic indicator determines...
19 KB (1,671 words) - 21:32, 19 June 2025
bond. Acid strengths also depend on the stability of the conjugate base. While the K a {\displaystyle K_{a}} value measures the tendency of an acidic solute...
19 KB (2,474 words) - 21:52, 17 April 2025
molecule, or ion, forming a conjugate acid. (The complementary process, when a proton is removed from a Brønsted–Lowry acid, is deprotonation.) Some examples...
4 KB (466 words) - 13:10, 27 June 2025
Neutralization (chemistry) (redirect from Acid-Base neutralization)
end-point the acid is completely neutralized so the analytical hydrogen ion concentration, TH, is zero and the concentration of the conjugate base, A−, is equal...
17 KB (2,128 words) - 01:06, 10 June 2025
are not bound to H+ (see Conjugate acid-base theory). It is α-ketoglutarate and itaconate, not α-ketoglutaric or itaconic acids, which activate OXGR1. In...
33 KB (4,235 words) - 01:55, 4 July 2024
supplement or pharmaceutical drug in some countries. Lipoate is the conjugate base of lipoic acid, and the most prevalent form of LA under physiological conditions...
49 KB (4,977 words) - 09:25, 15 June 2025
protons, the conjugate base of an acid may itself be acidic. Conjugate base A conjugate acid, within the Brønsted–Lowry acid–base theory, is a species...
279 KB (31,747 words) - 13:03, 24 June 2025
Oxyacid (redirect from Oxygen acid)
Both acetic acid and methanol contain C-O-H groups that can act as acids, but acetic acid is a far stronger acid because its conjugate base, acetate, can...
23 KB (1,918 words) - 05:17, 26 May 2025
symbol for acetic acid is AcOH (or HOAc), where Ac is the pseudoelement symbol representing the acetyl group CH3−C(=O)−; the conjugate base, acetate (CH3COO−)...
63 KB (6,653 words) - 01:13, 24 June 2025
10-methenyltetrahydrofolate intermediate. The conjugate base of formic acid, formate, also occurs widely in nature. An assay for formic acid in body fluids, designed for...
40 KB (4,123 words) - 16:36, 24 May 2025
Chemical reaction (redirect from Metal-acid reaction)
A − conjugated base + HB + conjugated acid {\displaystyle {\ce {{\underset {acid}{HA}}+{\underset {base}{B}}<=>{\underset {conjugated\ base}{A^{-}}}+{\underset...
66 KB (8,048 words) - 01:14, 16 June 2025
of a monobasic acid will be used to simplify the presentation. The equilibrium for protonation of the conjugate base, A− of the acid HA, may be written...
10 KB (1,422 words) - 13:15, 15 March 2025
organic chemistry, Lewis acid catalysis is the use of metal-based Lewis acids as catalysts for organic reactions. The acids act as an electron pair acceptor...
45 KB (5,111 words) - 12:29, 27 June 2025
β-Hydroxy β-methylbutyric acid (HMB), otherwise known as its conjugate base, β-hydroxy β-methylbutyrate, is a naturally produced substance in humans that...
106 KB (9,925 words) - 02:21, 16 June 2025
Sulfuric acid (American spelling and the preferred IUPAC name) or sulphuric acid (Commonwealth spelling), known in antiquity as oil of vitriol, is a mineral...
65 KB (7,178 words) - 18:08, 1 July 2025
reactivities, local diamagnetic shielding in aromatics, deshielding, and acid and base strengths. Murrell, J N (1955-11-01). "The Electronic Spectrum of Aromatic...
10 KB (1,144 words) - 01:12, 24 June 2025
anion with the formula N−3 and structure −N=N+=N−. It is the conjugate base of hydrazoic acid HN3. Organic azides are organic compounds with the formula...
19 KB (2,194 words) - 08:17, 24 May 2025
addicted to drugs. The butyrate or butanoate ion, C3H7COO−, is the conjugate base of butyric acid. It is the form found in biological systems at physiological...
65 KB (6,516 words) - 17:09, 2 July 2025
PH (redirect from Acid and base)
must also be considered. A weak acid or the conjugate acid of a weak base can be treated using the same formalism. Acid HA: HA ↽ − − ⇀ H + + A − {\displaystyle...
53 KB (6,539 words) - 06:31, 29 June 2025
Oxyanion (category Acid–base chemistry)
example of an acid–base reaction with the monomeric oxyanion acting as a base and the condensed oxyanion acting as its conjugate acid. The reverse reaction...
18 KB (2,163 words) - 14:15, 13 August 2024