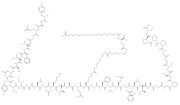
The European Medicines Agency (EMA) is an agency of the European Union (EU) in charge of the evaluation and supervision of pharmaceutical products. Prior...
43 KB (3,701 words) - 16:03, 18 May 2024
ensuring that medicines and medical devices work and are acceptably safe. The MHRA was formed in 2003 with the merger of the Medicines Control Agency (MCA) and...
24 KB (2,284 words) - 11:01, 6 March 2024
European Medicines Agency (EMA). 29 January 2020. Archived from the original on 14 August 2020. Retrieved 26 September 2020. "Wegovy EPAR". European Medicines...
66 KB (5,418 words) - 18:14, 27 May 2024
similar model to that of the European Medicines Agency, it is intended to have a wide scope covering medicines, traditional medicine, and medical devices. The...
20 KB (1,881 words) - 14:52, 6 June 2023
responsible for the regulatory activity of pharmaceuticals in Italy. European Medicines Agency Istituto Superiore di Sanità Official website v t e v t e...
464 bytes (37 words) - 18:24, 13 May 2024
© European Medicines Agency. Reproduction is authorized provided the source is acknowledged. "Dapagliflozin Viatris EPAR". European Medicines Agency. 4...
53 KB (4,939 words) - 05:15, 20 May 2024
Biosimilar (section European Union)
their lifecycle. Drug-related authorities such as the European Medicines Agency (EMA) of the European Union, the United States Food and Drug Administration...
95 KB (6,480 words) - 22:51, 13 May 2024
Approximately 180 people work at the agency; most are pharmacists and doctors. Regulation of therapeutic goods European Medicines Agency Official website v t e v...
3 KB (168 words) - 02:14, 9 December 2023
N-Nitrosodimethylamine (section European Union)
is © European Medicines Agency. Reproduction is authorized provided the source is acknowledged. "Nitrosamine impurities". European Medicines Agency (EMA)...
25 KB (2,390 words) - 04:57, 9 May 2024
and methods of analysis for medicines. These standards apply to medicines for both human and veterinary use. The European Pharmacopoeia has a legally...
11 KB (1,225 words) - 06:47, 24 April 2024
which is © European Medicines Agency. Reproduction is authorized provided the source is acknowledged. "Synflorix EPAR". European Medicines Agency (EMA). 17...
50 KB (4,208 words) - 01:34, 22 April 2024
vaccine has since been approved by several medicine agencies worldwide, such as the European Medicines Agency (EMA), and the Australian Therapeutic Goods...
214 KB (17,118 words) - 01:38, 27 May 2024
The European Directorate for the Quality of Medicines & HealthCare (EDQM) is a Directorate and partial agreement of the Council of Europe that traces...
33 KB (3,770 words) - 16:29, 27 April 2024
Adalimumab (category World Health Organization essential medicines)
European Medicines Agency (EMA). 17 September 2018. Archived from the original on 25 June 2019. Retrieved 18 February 2020. "Trudexa EPAR". European Medicines...
91 KB (6,940 words) - 04:46, 30 April 2024
European Medicines Agency in the European Union. In January 2018, the Committee for Medicinal Products for Human Use of the European Medicines Agency...
17 KB (1,585 words) - 19:15, 28 April 2024
May 2022. Text was copied from this source which is copyright European Medicines Agency. Reproduction is authorized provided the source is acknowledged...
14 KB (804 words) - 03:51, 31 March 2024
European Medicines Agency (EMA) started reviewing its position at the request of the French ANSM, which withdrew all pholcodine-containing medicines after...
22 KB (1,748 words) - 06:26, 18 May 2024
the brand name Zepbound. In November 2023, the UK Medicines and Healthcare products Regulatory Agency revised the indication for tirzepatide to include...
42 KB (3,392 words) - 23:29, 23 May 2024
Director of the European Medicines Agency (EMA) since November 2020. She is also the chairperson at the International Coalition of Medicines Regulatory Authorities...
6 KB (466 words) - 18:07, 13 December 2023
H-W-2495". European Medicines Agency (EMA). Archived from the original on 2 October 2018. Retrieved 28 June 2018. "Hexyon EPAR". European Medicines Agency (EMA)...
14 KB (1,021 words) - 18:26, 16 September 2023
which is copyright European Medicines Agency. Reproduction is authorized provided the source is acknowledged. "First-in-class medicine recommended for treatment...
17 KB (1,164 words) - 06:07, 10 March 2024
code itself is issued by the Medicines and Healthcare products Regulatory Agency in the UK and the European Medicines Agency. "Martin Lewis: A drugs bust...
1 KB (113 words) - 01:36, 16 December 2020
the European Medicines Agency in January 2023. On 20 July 2023, the Committee for Medicinal Products for Human Use (CHMP) of the European Medicines Agency...
13 KB (1,033 words) - 01:37, 18 May 2024
copyright European Medicines Agency. Reproduction is authorized provided the source is acknowledged. "Livogiva EPAR". European Medicines Agency (EMA). 26...
26 KB (1,978 words) - 17:03, 8 February 2024
population as opposed to Spaniards. An assessment report by the European Medicines Agency remarked that "the potential to induce agranulocytosis may be...
53 KB (4,614 words) - 04:59, 3 May 2024
Committee for Medicinal Products for Human Use (CHMP) of the European Medicines Agency (EMA) adopted a positive opinion, recommending the granting of...
25 KB (1,916 words) - 21:37, 20 December 2023
viral infections. The most common side effects reported by the European Medicines Agency (EMA) include injection-site reactions (such as redness, swelling...
22 KB (1,841 words) - 22:50, 30 April 2024
(Press release). European Medicines Agency. 6 January 2021. Archived from the original on 17 March 2021. Retrieved 6 January 2021. "European Commission authorises...
122 KB (11,028 words) - 02:32, 27 January 2024
European Medicines Agency (EMA). 14 September 2022. Retrieved 5 December 2022. Text was copied from this source which is copyright European Medicines...
14 KB (974 words) - 07:12, 14 January 2024
copyright European Medicines Agency. Reproduction is authorized provided the source is acknowledged. "New medicine for rare type of eye cancer". European Medicines...
13 KB (850 words) - 06:46, 10 March 2024