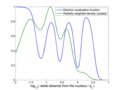
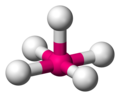
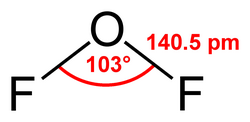
Valence shell electron pair repulsion (VSEPR) theory (/ˈvɛspər, vəˈsɛpər/ VESP-ər,: 410 və-SEP-ər) is a model used in chemistry to predict the geometry...
45 KB (4,059 words) - 23:42, 3 June 2025
levels of s and p orbitals. Bent's rule represents a modification of VSEPR theory for molecules of lower than ideal symmetry. For bonds with the larger...
38 KB (4,285 words) - 19:40, 22 May 2025
Molecular geometry (section VSEPR table)
angles in the table below are ideal angles from the simple VSEPR theory (pronounced "Vesper Theory")[citation needed], followed by the actual angle for the...
23 KB (2,301 words) - 04:26, 11 May 2025
Chemical bond (redirect from Bonding theory)
polarity of bonds. The octet rule and VSEPR theory are examples. More sophisticated theories are valence bond theory, which includes orbital hybridization...
40 KB (4,878 words) - 16:35, 25 May 2025
pair is a concept used in valence shell electron pair repulsion theory (VSEPR theory) which explains the shapes of molecules. They are also referred to...
23 KB (2,957 words) - 08:07, 16 March 2025
and methylene (CH2). This geometry is almost always consistent with VSEPR theory, which usually explains non-collinearity of atoms with a presence of...
3 KB (328 words) - 03:49, 14 February 2025
above and below the plane (axial or apical positions). According to the VSEPR theory of molecular geometry, an axial position is more crowded because an axial...
6 KB (683 words) - 15:25, 7 February 2023
geometry are sometimes described as sp3 hybridized. The AXE method for VSEPR theory states that the classification is AX3E1. The nitrogen in ammonia has...
4 KB (317 words) - 18:50, 10 December 2024
1918 to the 1920s and from 1939 to 1945 Vespa Vespers (disambiguation) VSEPR theory in chemistry, often pronounced "Vesper" This disambiguation page lists...
3 KB (390 words) - 04:26, 8 May 2025
and lone pairs, in what has been called "a faithful visualization of VSEPR theory in action". Another feature of the ELF is that it is invariant concerning...
7 KB (912 words) - 15:22, 25 May 2025
example of steric activity is SnCl2, which is bent in accordance with VSEPR theory. Some examples where the lone pair appears to be inactive are bismuth(III)...
9 KB (1,153 words) - 18:24, 17 February 2025
Orbital hybridisation (redirect from Hybridization theory)
repulsion (VSEPR) theory, which can be used to predict molecular geometry based on empirical rules rather than on valence-bond or orbital theories. As the...
33 KB (3,204 words) - 20:53, 19 May 2025
Quantum chemistry (section Valence bond theory)
approaches are used, including semi-empirical methods, density functional theory, Hartree–Fock calculations, quantum Monte Carlo methods, and coupled cluster...
20 KB (2,260 words) - 13:17, 23 May 2025
the crystal, the species adopts a pyramidal structure, in accord with VSEPR theory. Bismuth chloride can be synthesized directly by passing chlorine over...
8 KB (592 words) - 19:40, 7 March 2025
T-shaped molecules are the halogen trifluorides, such as ClF3. According to VSEPR theory, T-shaped geometry results when three ligands and two lone pairs of electrons...
4 KB (377 words) - 18:50, 10 December 2024
and trigonal pyramidal shape (steric number 4 with one lone pair; see VSEPR theory). When the two organic residues are dissimilar, the sulfur atom is a...
14 KB (1,425 words) - 15:54, 17 December 2024
compounds that have a stereochemically-active lone pair, as described by VSEPR theory. Certain compounds crystallize in both the trigonal bipyramidal and the...
4 KB (300 words) - 13:26, 23 December 2024
stereochemistry is denoted in skeletal formulae. Solid-state chemistry VSEPR theory Nuclear Overhauser effect, a method in nuclear magnetic resonance spectroscopy...
12 KB (1,263 words) - 22:18, 24 May 2025
qualitative theories. Such theories are easier to learn as they require little background in quantum theory. Within main group compounds, VSEPR theory powerfully...
30 KB (3,224 words) - 15:33, 23 May 2025
should feature the strongest bonding of all group 17 monofluorides. VSEPR theory predicts a bent-T-shaped molecular geometry for the group 17 trifluorides...
70 KB (11,063 words) - 17:53, 26 May 2025
059. Nash, Clinton S.; Bursten, Bruce E. (1999). "Spin-Orbit Effects, VSEPR Theory, and the Electronic Structures of Heavy and Superheavy Group IVA Hydrides...
64 KB (10,403 words) - 17:05, 24 May 2025
a non-bonded lone pair on the sulfur, so the structure predicted by VSEPR theory is trigonal pyramidal, as in ammonia (NH3). In the hybrid resonance structure...
20 KB (1,829 words) - 04:22, 17 March 2025
tetrahedral molecular geometry of the sulfate ion is as predicted by VSEPR theory. The first description of the bonding in modern terms was by Gilbert...
24 KB (4,482 words) - 17:06, 23 May 2025
square planar, consistent with VSEPR theory for four ligands and two lone pairs (or AX4E2 in the notation of VSEPR theory). The XeO2 network does not share...
5 KB (433 words) - 00:43, 3 January 2025
fourth apex of an approximately regular tetrahedron, as predicted by the VSEPR theory. This orbital is not participating in covalent bonding; it is electron-rich...
24 KB (2,763 words) - 11:45, 25 May 2025
as has been confirmed by neutron diffraction studies. According to VSEPR theory, in addition to four fluoride ligands, the xenon center has two lone...
11 KB (952 words) - 07:42, 7 March 2025
difluoride is a chemical compound with the formula OF2. As predicted by VSEPR theory, the molecule adopts a bent molecular geometry.[citation needed] It is...
9 KB (729 words) - 14:55, 6 March 2025
shared and unshared pairs). This concept was later developed into the VSEPR theory of molecular geometry. 20世紀日本人名事典. "槌田 龍太郎(ツチダ リュウタロウ)とは? 意味や使い方". コトバンク...
2 KB (175 words) - 01:29, 28 May 2025
as Be2C. The gas phase is noteworthy for failing the predictions of VSEPR theory; the CaF2 molecule is not linear like MgF2, but bent with a bond angle...
10 KB (843 words) - 13:32, 4 February 2025
crystallography has been used to determine its structure; as can be predicted by VSEPR theory, it adopts a T-shaped geometry about the central iodine atom. Iodobenzene...
5 KB (422 words) - 13:38, 5 April 2025