The vapor–liquid–solid method (VLS) is a mechanism for the growth of one-dimensional structures, such as nanowires, from chemical vapor deposition. The...
17 KB (2,304 words) - 11:18, 22 October 2024
particles escaping from the liquid (or solid) in equilibrium with those in a coexisting vapor phase. A substance with a high vapor pressure at normal temperatures...
26 KB (3,008 words) - 14:31, 18 April 2025
VLS may refer to: Vapor-Liquid-Solid method, a method of growing nanocrystals Vermont Law School Vertical Launching System for firing missiles Von Luschan's...
524 bytes (96 words) - 21:53, 23 January 2021
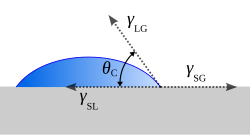
Contact angle (section The static sessile drop method)
between a liquid surface and a solid surface where they meet. More specifically, it is the angle between the surface tangent on the liquid–vapor interface...
30 KB (3,998 words) - 12:19, 26 December 2024
may also be formed by the condensation of a vapor or by atomization of a larger mass of solid. Water vapor will condense into droplets depending on the...
17 KB (1,993 words) - 02:22, 30 April 2025
Gas chromatography (redirect from Vapor-phase chromatography)
mixture. Gas chromatography is also sometimes known as vapor-phase chromatography (VPC), or gas–liquid partition chromatography (GLPC). These alternative...
38 KB (5,022 words) - 19:44, 1 May 2025
Volatility (chemistry) (redirect from Volatile liquids)
is more likely to be a liquid or solid. Volatility can also describe the tendency of a vapor to condense into a liquid or solid; less volatile substances...
10 KB (1,179 words) - 12:01, 23 April 2025
temperature and pressure at which solid, liquid, and gaseous water can coexist in a stable equilibrium (273.16 K and a partial vapor pressure of 611.657 Pa). The...
22 KB (2,517 words) - 20:50, 4 March 2025
Solution (chemistry) (redirect from Liquid solution)
saturation vapor pressure at a given temperature is reached, vapor excess condenses into the liquid state. Liquids dissolve gases, other liquids, and solids. An...
10 KB (1,290 words) - 23:34, 15 December 2024
Flash evaporation (category Gas-liquid separation)
Flash evaporation (or partial evaporation) is the partial vapor that occurs when a saturated liquid stream undergoes a reduction in pressure by passing through...
11 KB (1,321 words) - 06:26, 12 June 2024
richer phase diagrams, discussed below). The commonly known phases solid, liquid and vapor are separated by phase boundaries, i.e. pressure–temperature combinations...
21 KB (1,980 words) - 10:58, 28 October 2024
Vapor-compression evaporation is the evaporation method by which a blower, compressor or jet ejector is used to compress, and thus, increase the pressure...
12 KB (1,598 words) - 19:01, 7 March 2025
atmospheric water vapor and ice. However, using current methods of astronomical spectroscopy it is substantially more difficult to detect liquid water on terrestrial...
74 KB (7,535 words) - 21:41, 4 May 2025
Chemical vapor deposition (CVD) is a vacuum deposition method used to produce high-quality, and high-performance, solid materials. The process is often...
42 KB (5,024 words) - 15:12, 30 March 2025
vapor. This is evident from the longevity of the ice that composes Saturn's rings. Liquids can form solutions with gases, solids, and other liquids....
61 KB (7,399 words) - 21:48, 4 April 2025
Heat pipe (redirect from Vapor Chamber Technology)
two solid interfaces. At the hot interface of a heat pipe, a volatile liquid in contact with a thermally conductive solid surface turns into a vapor by...
56 KB (6,878 words) - 17:36, 8 May 2025
above-mentioned techniques, at certain conditions, and also with the vapor–liquid–solid method. The synthesis is typically carried out at temperatures of about...
85 KB (9,287 words) - 21:36, 10 May 2025
others led to widespread use of mercury-vapor lamps for general lighting. The mercury in the tube is a liquid at normal temperatures. It needs to be vaporized...
23 KB (2,781 words) - 16:29, 31 March 2025
effect distillation, mechanical vapor compression, crystallization, and condensate recovery. ZLD plants produce solid waste. ZLD processes begin with...
7 KB (685 words) - 09:29, 11 August 2024
Aerogel (redirect from Solid smoke)
which the liquid component for the gel has been replaced with a gas, without significant collapse of the gel structure. The result is a solid with extremely...
74 KB (8,158 words) - 19:20, 19 April 2025
Distillation (redirect from Method by distillation)
liquid boiling points differ greatly (rule of thumb is 25 °C) or when separating liquids from non-volatile solids or oils. For these cases, the vapor...
72 KB (8,983 words) - 15:12, 29 March 2025
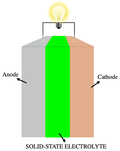
between the electrodes, instead of the liquid or gel polymer electrolytes found in conventional batteries. Solid-state batteries theoretically offer much...
100 KB (10,589 words) - 16:10, 9 May 2025
be produced from the evaporation or boiling of liquid water or from the sublimation of ice. Water vapor is transparent, like most constituents of the atmosphere...
60 KB (6,470 words) - 03:39, 27 March 2025
the transition of a substance directly from the solid to the gas state, without passing through the liquid state. The verb form of sublimation is sublime...
23 KB (2,429 words) - 02:37, 7 April 2025
Surface energy (section Deformed solid)
changes in vapor pressure caused by liquids with curved surfaces. The cause for this change in vapor pressure is the Laplace pressure. The vapor pressure...
33 KB (4,063 words) - 15:27, 29 March 2025
Epitaxy (redirect from Liquid phase epitaxy)
saturation and chemisorption. Liquid-phase epitaxy (LPE) is a method to grow semiconductor crystal layers from the melt on solid substrates. This happens at...
30 KB (3,624 words) - 17:18, 27 February 2025
Crystallization (redirect from Crystallization methods)
is also a chemical solid–liquid separation technique, in which mass transfer of a solute from the liquid solution to a pure solid crystalline phase occurs...
30 KB (3,886 words) - 01:43, 23 April 2025
Aerosol (redirect from Liquid aerosol)
An aerosol is a suspension of fine solid particles or liquid droplets in air or another gas. Aerosols can be generated from natural or human causes. The...
47 KB (5,933 words) - 17:01, 13 April 2025
Water (redirect from Liquid water)
diagram (see figure), there are curves separating solid from vapor, vapor from liquid, and liquid from solid. These meet at a single point called the triple...
172 KB (20,576 words) - 16:32, 4 May 2025
surface tension, the vapor pressure for small droplets of liquid in suspension is greater than standard vapor pressure of that same liquid when the interface...
72 KB (8,772 words) - 13:30, 3 April 2025