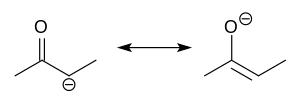
In organic chemistry, enolates are organic anions derived from the deprotonation of carbonyl (RR'C=O) compounds. Rarely isolated, they are widely used...
21 KB (2,467 words) - 09:11, 19 May 2025
Enol (redirect from Enolate ion)
Deprotonation of enolizable ketones, aldehydes, and esters gives enolates. Enolates can be trapped by the addition of electrophiles at oxygen. Silylation...
14 KB (1,159 words) - 01:14, 13 March 2025
substitution of an α-hydrogen by an electrophile through either an enol or enolate ion intermediate. Because their double bonds are electron rich, enols behave...
7 KB (982 words) - 20:11, 11 November 2024
Organolithium reagent (section Enolate formation)
enantiomer. Lithium enolates are formed through deprotonation of a C−H bond α to the carbonyl group by an organolithium species. Lithium enolates are widely used...
55 KB (5,955 words) - 23:00, 13 March 2025
Aldol reactions (section Enolate mechanism)
or ketones. Aldol addition or aldolization refers to the addition of an enolate or enolation as a nucleophile to a carbonyl moiety as an electrophile....
9 KB (957 words) - 15:23, 19 January 2025
enolizable (have an α-proton and be able to undergo deprotonation to form the enolate anion). There are a number of different combinations of enolizable and...
7 KB (633 words) - 15:57, 24 May 2025
case mediated by the enolate or the proton source. In the deprotonation of an unsymmetrical ketone, the kinetic product is the enolate resulting from removal...
21 KB (2,751 words) - 18:12, 1 November 2024
in which context it is also known as Mander's reagent. When a lithium enolate is generated in diethyl ether or methyl t-butyl ether, treatment with Mander's...
3 KB (155 words) - 15:38, 25 October 2024
reaction or Michael 1,4 addition is a reaction between a Michael donor (an enolate or other nucleophile) and a Michael acceptor (usually an α,β-unsaturated...
27 KB (2,826 words) - 19:31, 22 May 2025
tertiary alcohols. Esters also react readily with enolates. In the Claisen condensation, an enolate of one ester (1) will attack the carbonyl group of...
45 KB (4,636 words) - 08:55, 18 June 2025
In its usual form, it involves the nucleophilic addition of a ketone enolate to an aldehyde to form a β-hydroxy ketone, or aldol (aldehyde + alcohol)...
18 KB (1,816 words) - 08:49, 7 June 2025
compounds that share the common functional group R3Si−O−CR=CR2, composed of an enolate (R3C−O−R) bonded to a silane (SiR4) through its oxygen end and an ethene...
13 KB (1,369 words) - 22:56, 11 March 2025
Acetic anhydride (section Enolate formation)
(CH3CO)2O + H2O → 2 CH3COOH Acetic anhydride forms the enolate in the presence of acetate as base. The enolate can be trapped by condenation with benzaldehyde...
17 KB (1,460 words) - 19:50, 22 April 2025
using a lithium enolate compared to 97:3 using a dibutylboron enolate. Where the counterion determines stereoinduction strength, the enolate isomer determines...
40 KB (4,256 words) - 12:17, 6 June 2025
strong base such as lithium diisopropylamide selectively furnishes the (Z)-enolate, which can undergo stereoselective alkylation. Alkylation of an oxazolidinone...
34 KB (3,726 words) - 10:21, 15 March 2025
is readily substituted by nucleophiles such as amines, phenoxides, and enolates, giving sulfonamides, aryl nonaflates, and alkenyl nonaflates, respectively...
11 KB (1,192 words) - 18:52, 27 May 2025
withdrawing group. Z must be powerful enough to facilitate deprotonation to the enolate ion even with a mild base. Using a strong base in this reaction would induce...
7 KB (729 words) - 10:15, 12 February 2025
2,5-Diketopiperazine (section Alkylation of enolates)
5-diketopiperazine ring by enolate acylation was used in the construction of the 2,5-diketopiperazine ring in 11 by intramolecular cyclization of the enolate of 10 onto...
22 KB (2,289 words) - 02:24, 9 June 2025
Silylation (section Enolate Trapping)
groups to solids. Silyl groups are commonly used for: alcohol protection, enolate trapping, gas chromatography, electron-impact mass spectrometry (EI-MS)...
10 KB (1,072 words) - 16:26, 21 May 2025
Conia-ene reaction (section Enolate activation)
form a metal-stabilized enolate, which then attacks the tethered alkyne and transfers the metal cation. An early example of enolate activation was reported...
16 KB (1,805 words) - 20:22, 22 May 2025
Oxaziridine (redirect from Sulfonyloxaziridine enolate oxidation)
hydrazine: Me(Et)CONH + NH3 → Me(Et)C=NNH2 + H2O N-sulfonyloxaziridines oxidize enolates to acyloins with high chiral induction, better than (e.g.) MoOPH. Chiral...
24 KB (2,194 words) - 12:34, 6 June 2025
initiator, it can replace some organotin compounds. It reacts with metal enolates, yielding enoxytriethylborates that can be alkylated at the α-carbon atom...
12 KB (1,010 words) - 14:39, 23 May 2025
because this species exists mainly as the monoenol CH3C(O)CH=C(OH)CH3. Its enolate is a common ligand in coordination chemistry. Ketones containing alkene...
24 KB (2,895 words) - 18:04, 2 June 2025
'Reformatsky enolate', is prepared by treating an alpha-halo ester with zinc dust. Reformatsky enolates are less reactive than lithium enolates or Grignard...
13 KB (1,275 words) - 23:31, 13 March 2025
with base to form 1,3-diketones. This rearrangement reaction proceeds via enolate formation followed by acyl transfer. It is named after the scientists Wilson...
5 KB (550 words) - 09:56, 24 May 2025
cannot form an enolate, and the benzaldehyde is much more electrophilic than any unenolized acetophenone in solution. Therefore, the enolate formed from...
4 KB (488 words) - 13:28, 23 December 2024
in order to generate the thiophenol from the corresponding aryl halide. Enolates and other similar carbon nucleophiles can also be coupled to produce α-aryl...
31 KB (3,483 words) - 13:42, 28 March 2025
peroxide enolate (Figure 3). All of the reactants associate with the polyleucine catalyst prior to reaction to form the hydroperoxide enolate intermediate...
21 KB (2,152 words) - 06:44, 2 December 2024
deprotonated at the kinetic site. This enolate may then encounter other ketones and the thermodynamic enolate will form through the exchange of protons...
8 KB (780 words) - 23:21, 20 December 2024
salt at –78 °C to give a chelated enolate as intermediate. While different metal salts can be used to form the enolate, the use of zinc chloride results...
24 KB (2,532 words) - 12:24, 5 April 2025