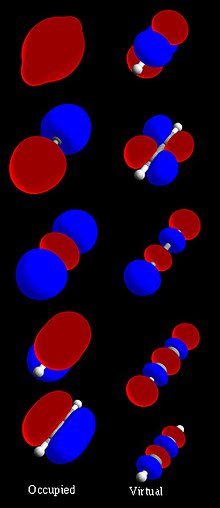
mechanics, an atomic orbital (/ˈɔːrbɪtəl/) is a function describing the location and wave-like behavior of an electron in an atom. This function describes...
83 KB (10,720 words) - 04:42, 17 May 2024
inferred" that a carbon atom would form three bonds at right angles (using p orbitals) and a fourth weaker bond using the s orbital in some arbitrary direction...
33 KB (3,164 words) - 10:14, 24 April 2024
A hydrogen atom is an atom of the chemical element hydrogen. The electrically neutral atom contains a single positively charged proton and a single negatively...
38 KB (5,789 words) - 07:10, 19 April 2024
amplitude. In an isolated atom, the orbital electrons' location is determined by functions called atomic orbitals. When multiple atoms combine chemically into...
34 KB (4,340 words) - 17:17, 11 May 2024
A hydrogen-like atom (or hydrogenic atom) is any atom or ion with a single valence electron. These atoms are isoelectronic with hydrogen. Examples of hydrogen-like...
28 KB (5,288 words) - 21:06, 20 February 2024
Azimuthal quantum number (redirect from Orbital quantum number)
number for an atomic orbital that determines its orbital angular momentum and describes aspects of the angular shape of the orbital. The azimuthal quantum...
19 KB (2,121 words) - 15:28, 24 April 2024
Electron configuration (redirect from Orbital ordering)
precedes each orbital letter (helium's electron configuration is 1s2, therefore n = 1, and the orbital contains two electrons). An atom's nth electron...
60 KB (6,147 words) - 22:00, 18 May 2024
"The Quantum Atom". University of Florida. Archived from the original on 7 December 2006. Manthey, David (2001). "Atomic Orbitals". Orbital Central. Archived...
123 KB (12,596 words) - 20:39, 18 May 2024
electrons as molecular orbitals that surround two or more atoms in a molecule and contain valence electrons between atoms. Molecular orbital theory revolutionized...
22 KB (2,943 words) - 18:31, 27 April 2024
Bohr model (redirect from Atom/Bohr model)
model of the atom, presented by Niels Bohr and Ernest Rutherford in 1913. It consists of a small, dense nucleus surrounded by orbiting electrons. It...
64 KB (9,057 words) - 02:14, 6 May 2024
the orbital electrons are replaced by a negatively charged hadron. Possible hadrons include mesons such as the pion or kaon, yielding a pionic atom or...
12 KB (1,471 words) - 10:44, 21 May 2024
History of atomic theory (redirect from Atom theory)
scientific theory that matter is composed of particles called atoms. The definition of the word "atom" has changed over the years in response to scientific discoveries...
67 KB (8,832 words) - 20:43, 21 May 2024
Magnetic quantum number (redirect from Orbital magnetic quantum number)
axis in space. The orbital magnetic quantum number (ml or m) distinguishes the orbitals available within a given subshell of an atom. It specifies the...
10 KB (1,208 words) - 03:22, 10 December 2023
atom. In spite of its shortcomings, the Bohr model of the atom is useful in explaining these properties. Classically, an electron in a circular orbit...
51 KB (5,769 words) - 06:37, 19 May 2024
Pi bond (redirect from Pi bonding molecular orbital)
orbital on one atom overlap with two lobes of an orbital on another atom, and in which this overlap occurs laterally. Each of these atomic orbitals has...
7 KB (816 words) - 06:21, 24 December 2023
Energy level (redirect from Quantized energy levels of atoms)
as a circular orbit around an atom, where the number of wavelengths gives the type of atomic orbital (0 for s-orbitals, 1 for p-orbitals and so on). Elementary...
22 KB (2,831 words) - 03:08, 12 February 2024
molecular orbital diagram, or MO diagram, is a qualitative descriptive tool explaining chemical bonding in molecules in terms of molecular orbital theory...
38 KB (4,966 words) - 02:30, 13 March 2024
bond order between the involved atoms. Non-bonding orbitals are often designated by the letter n in molecular orbital diagrams and electron transition...
5 KB (660 words) - 20:31, 24 June 2023
which includes orbital hybridization and resonance, and molecular orbital theory which includes the linear combination of atomic orbitals and ligand field...
40 KB (4,876 words) - 22:33, 22 February 2024
given atom. In chemical reactions, orbital wavefunctions are modified, i.e. the electron cloud shape is changed, according to the type of atoms participating...
6 KB (781 words) - 00:03, 6 April 2023
Angular momentum coupling (redirect from Orbit-orbit coupling)
its spin and its orbital angular momentum. If the spin has half-integer values, such as 1/2 for an electron, then the total (orbital plus spin) angular...
17 KB (2,228 words) - 20:09, 21 February 2024
Carbon–carbon bond (redirect from Quaternary carbon atom)
hybridized orbital from each of the carbon atoms. In ethane, the orbitals are sp3-hybridized orbitals, but single bonds formed between carbon atoms with other...
9 KB (1,043 words) - 22:45, 2 February 2024
Sigma bond (redirect from Sigma molecular orbital)
corresponding antibonding, or σ* orbital, is defined by the presence of one nodal plane between the two bonded atoms. Sigma bonds are the strongest type...
8 KB (909 words) - 16:39, 8 November 2023
Quantum number (redirect from Quantum numbers with spin-orbit interaction)
contains only one orbital, and therefore the mℓ of an electron in an s orbital will always be 0. The p subshell (ℓ = 1) contains three orbitals, so the mℓ of...
30 KB (3,122 words) - 23:06, 1 May 2024
Atomic nucleus (redirect from Nucleus of an atom)
and two neutrons separately occupy 1s orbitals analogous to the 1s orbital for the two electrons in the helium atom, and achieve unusual stability for the...
34 KB (3,994 words) - 07:45, 8 April 2024
An electron orbital may refer to: An atomic orbital, describing the behaviour of an electron in an atom A molecular orbital, describing the behaviour...
472 bytes (94 words) - 08:06, 27 October 2017
Delocalized electron (section Molecular orbitals)
orbitals, sharing the electrons uniformly among all five atoms. There are two orbital levels, a bonding molecular orbital formed from the 2s orbital on...
4 KB (448 words) - 09:16, 12 February 2024
theoretical chemistry, an antibonding orbital is a type of molecular orbital that weakens the chemical bond between two atoms and helps to raise the energy of...
7 KB (880 words) - 07:57, 10 May 2023
bonding orbital is used in molecular orbital (MO) theory to describe the attractive interactions between the atomic orbitals of two or more atoms in a molecule...
9 KB (1,093 words) - 22:34, 24 September 2022
molecular orbitals in molecular orbital theory and resonance of sigma bonds in valence bond theory. In three-center two-electron bonds ("3c–2e") three atoms share...
28 KB (3,654 words) - 07:13, 1 April 2024