The bond-dissociation energy (BDE, D0, or DH°) is one measure of the strength of a chemical bond A−B. It can be defined as the standard enthalpy change...
23 KB (2,316 words) - 21:30, 13 November 2023
average bond enthalpy, or bond strength. IUPAC defines bond energy as the average value of the gas-phase bond-dissociation energy (usually at a temperature...
9 KB (1,318 words) - 02:44, 29 April 2024
energy of a sigma bond is the energy required for homolytic dissociation, but the actual excitation energy may be higher than the bond-dissociation energy...
6 KB (696 words) - 03:07, 24 August 2023
Homolysis (chemistry) (redirect from Homolysis bond cleavage)
stabilize the free radical, the energy of the SOMO will be lowered, as will the bond dissociation energy. Bond dissociation energy is determined by multiple...
8 KB (992 words) - 18:20, 26 April 2024
generally stronger; the double bond of ethylene and triple bond of acetylene have been determined to have bond dissociation energies of 174 and 230 kcal/mol...
9 KB (1,043 words) - 22:45, 2 February 2024
calculations. Bond energy and bond-dissociation energy Gravitational binding energy Ionization energy (binding energy of one electron) Nuclear binding energy Quantum...
14 KB (1,389 words) - 20:37, 12 March 2024
Heterolysis (chemistry) (redirect from Heterolytic bond cleavage)
The energy required to break the bond is called the heterolytic bond dissociation energy, which is similar (but not equivalent) to homolytic bond dissociation...
5 KB (641 words) - 21:53, 3 February 2024
from the original on 29 August 2016. Yu-Ran Luo and Jin-Pei Cheng "Bond Dissociation Energies" in CRC Handbook of Chemistry and Physics, 96th Edition...
6 KB (534 words) - 15:22, 31 January 2024
Disulfide (redirect from Disulfide bond)
groups to encourage the dynamic dissociation of the S−S bond; these chemistries can result in the bond dissociation energy being reduced to half (or even...
37 KB (4,480 words) - 21:02, 10 April 2024
reactive diatomic molecule diphosphorus, which has roughly half the bond-dissociation energy of dinitrogen. Huber, K. P.; Herzberg, G. (1979). Molecular Spectra...
5 KB (516 words) - 14:46, 15 March 2024
value). Bond-dissociation energy Photodissociation, dissociation of molecules by photons (light, gamma rays, x-rays) Radiolysis, dissociation of molecules...
9 KB (1,309 words) - 19:07, 16 February 2024
metal–metal bond lengths in these M2 molecules increase down the group from Ca2 to Ubn2. On the other hand, their metal–metal bond-dissociation energies generally...
51 KB (8,779 words) - 11:04, 15 May 2024
Collision-induced dissociation (CID), also known as collisionally activated dissociation (CAD), is a mass spectrometry technique to induce fragmentation...
11 KB (1,347 words) - 09:38, 10 April 2024
benzylic positions is attributed to the low bond dissociation energy for benzylic C−H bonds. Specifically, the bond C6H5CH2−H is about 10–15% weaker than other...
15 KB (1,604 words) - 15:45, 3 December 2023
bond energy. For heteronuclear bonds, A−X, Pauling estimated the covalent contribution to the bond dissociation energy as being the mean of the bond dissociation...
12 KB (1,386 words) - 13:37, 13 May 2024
metal–metal bond-dissociation energies. The Uue–Uue bond should be slightly stronger than the K–K bond. From these M2 dissociation energies, the enthalpy...
42 KB (8,085 words) - 09:47, 15 May 2024
radical through homolytic dissociation of the carbon-cobalt bond. This bond is exceptionally weak, with a bond dissociation energy of 31 kcal/mol, which is...
5 KB (341 words) - 19:07, 25 March 2024
Organosulfur chemistry (redirect from Carbon-sulfur bond)
energy decreases to 73 kcal/mol (305 kJ/mol). The single carbon to oxygen bond is shorter than that of the C−C bond. The bond dissociation energies for...
26 KB (3,035 words) - 21:07, 2 May 2024
recorded ionization energy for a stable chemical compound. Bond-dissociation energy, the measure of the strength of a chemical bond calculated through...
51 KB (5,883 words) - 15:10, 14 May 2024
Carbon–fluorine bonds can have a bond dissociation energy (BDE) of up to 130 kcal/mol. The BDE (strength of the bond) of C–F is higher than other carbon–halogen...
14 KB (1,566 words) - 07:41, 19 December 2023
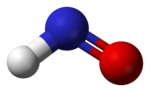
isoelectronic with dioxygen. The bond dissociation energy of H−NO is 49.5 kcal/mol (207 kJ/mol), which is unusually weak for a bond to the hydrogen atom. Nitroxyl...
9 KB (898 words) - 05:21, 15 April 2024
therefore, very reactive with a bond-dissociation energy (117 kcal/mol or 490 kJ/mol) half that of dinitrogen. The bond distance has been measured at 1.8934...
7 KB (631 words) - 06:17, 8 September 2023
participate in bond formation the bond is shorter. Bond length is also inversely related to bond strength and the bond dissociation energy: all other factors...
11 KB (1,125 words) - 00:59, 15 March 2024
etc. Bond-dissociation energy is the energy required to break one bond of a molecule or ion, usually separating an atom or atoms. Binding energy Look...
2 KB (248 words) - 11:41, 12 February 2022
Allyl group (section Bonding)
allylic. The bond dissociation energy of C−H bonds on a doubly allylic centre is about 10% less than the bond dissociation energy of a C−H bond that is singly...
13 KB (1,366 words) - 12:56, 2 May 2024
Diphenylmethane (section Reactivity of the C-H bond)
(C6H5)2CH–H bond, which has a bond dissociation energy of 82 kcal mol−1 (340 kJ mol−1). This is well below the published bond dissociation energies for comparable...
7 KB (457 words) - 18:05, 11 July 2022
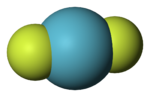
fluoride. In comparison, the dissociation of difluorine to atomic fluorine requires cleaving a F–F bond with a bond dissociation energy of 36 kcal/mol. Consequently...
13 KB (1,486 words) - 20:36, 14 May 2024
low energy. For example, the photon energy of visible light is about 1.8 to 3.2 eV. Similarly, the bond-dissociation energy of a carbon-carbon bond is...
9 KB (1,136 words) - 16:15, 10 May 2024
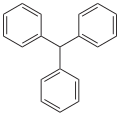
Triphenylmethane (section Reactions of C-H bond)
benzaldehyde and phosphorus pentachloride. The Ph3C-H bond is relatively weak, with a bond dissociation energy (BDE) of 81 kcal/mol, or about 24 kcal/mol less...
8 KB (613 words) - 21:48, 26 April 2024
Si-H bond. The compound is notable as having a weak Si-H bond, with a bond dissociation energy estimated at 84 kcal/mol. For comparison, the Si-H bond in...
4 KB (344 words) - 10:45, 1 March 2024