A buffer solution is a solution where the pH does not change significantly on dilution or if an acid or base is added at constant temperature. Its pH... 22 KB (2,334 words) - 20:33, 27 February 2024 |
Phosphate-buffered saline (PBS) is a buffer solution (pH ~ 7.4) commonly used in biological research. It is a water-based salt solution containing disodium... 9 KB (808 words) - 01:53, 17 April 2024 |
 | Intravenous therapy (redirect from Intravenous buffer solution) base solution to which medications are added also has some buffering effect. Another solution administered intravenously as a buffering solution is sodium... 60 KB (7,182 words) - 01:54, 30 March 2024 |
TAE buffer is a buffer solution containing a mixture of Tris base, acetic acid and EDTA. In molecular biology, it is used in agarose electrophoresis typically... 4 KB (540 words) - 18:20, 28 October 2023 |
TE buffer is a commonly used buffer solution in molecular biology, especially in procedures involving DNA, cDNA or RNA. "TE" is derived from its components:... 4 KB (552 words) - 15:35, 7 July 2023 |
 | PH (redirect from Neutral solution) standard solution, and the reading on a pH meter is adjusted to be equal to the standard buffer's value. The reading from a second standard buffer solution is... 49 KB (6,139 words) - 02:10, 24 April 2024 |
Tris/Borate/EDTA, is a buffer solution containing a mixture of Tris base, boric acid and EDTA. In molecular biology, TBE and TAE buffers are often used in... 2 KB (298 words) - 03:21, 3 March 2023 |
 | Sodium acetate (section Buffer solution) eat at low concentration. A solution of sodium acetate (a basic salt of acetic acid) and acetic acid can act as a buffer to keep a relatively constant... 17 KB (1,261 words) - 10:41, 29 April 2024 |
McIlvaine buffer is a buffer solution composed of citric acid and disodium hydrogen phosphate, also known as citrate-phosphate buffer. It was introduced... 3 KB (198 words) - 11:35, 8 July 2022 |
Look up buffer in Wiktionary, the free dictionary. Buffer may refer to: Buffer gas, an inert or nonflammable gas Buffer solution, a solution used to prevent... 2 KB (293 words) - 03:17, 12 December 2023 |
A lysis buffer is a buffer solution used for the purpose of breaking open cells for use in molecular biology experiments that analyze the labile macromolecules... 15 KB (1,825 words) - 04:05, 23 December 2023 |
 | value of 6.15 at 20 °C. The pH (and pKa at ionic strength I≠0) of the buffer solution changes with concentration and temperature, and this effect may be... 6 KB (475 words) - 01:46, 24 February 2024 |
Producer–consumer problem (redirect from Bounded-buffer problem) known as the bounded-buffer problem) is a family of problems described by Edsger W. Dijkstra since 1965. Dijkstra found the solution for the producer-consumer... 17 KB (2,182 words) - 20:08, 2 January 2024 |
 | Tris (redirect from Tris buffer) and molecular biology as a component of buffer solutions such as in TAE and TBE buffers, especially for solutions of nucleic acids. It contains a primary... 11 KB (980 words) - 12:47, 5 April 2024 |
vial and capillary are filled with an electrolyte such as an aqueous buffer solution. To introduce the sample, the capillary inlet is placed into a vial... 36 KB (4,675 words) - 10:51, 16 January 2024 |
Robinson (1904–1979). Buffer solution Good's buffers Mongay, Carlos; Cerdà, Víctor (January 1974). "A Britton-Robinson Buffer of Known Ionic Strength"... 2 KB (272 words) - 18:21, 28 December 2023 |
Good's buffers (also Good buffers) are twenty buffering agents for biochemical and biological research selected and described by Norman Good and colleagues... 8 KB (852 words) - 23:28, 3 April 2024 |
vary, and the resulting solution would still be referred to as "borate buffered saline". Borate concentration (giving buffering capacity) can vary from... 2 KB (270 words) - 22:51, 21 December 2023 |
biochemistry and molecular biology, saline-sodium citrate (SSC) buffer is used as a hybridization buffer, to control stringency for washing steps in protocols for... 992 bytes (101 words) - 22:18, 16 January 2022 |
Henderson–Hasselbalch equation (redirect from Buffer equation) Henderson–Hasselbalch equation can be used to estimate the pH of a buffer solution by approximating the actual concentration ratio as the ratio of the... 10 KB (1,425 words) - 04:13, 30 March 2024 |
Total ionic strength adjustment buffer (TISAB) is a buffer solution which increases the ionic strength of a solution to a relatively high level. This is... 3 KB (474 words) - 17:46, 28 May 2022 |
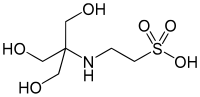 | to make buffer solutions. It has a pKa value of 7.550 (I=0, 25°C). It is one of the Good's buffers and can be used to make buffer solutions in the pH... 2 KB (90 words) - 22:18, 16 January 2022 |
 | Ammonium acetate (redirect from Ammonium acetate solution) acid to create a buffer solution. Ammonium acetate is volatile at low pressures. Because of this, it has been used to replace cell buffers that contain non-volatile... 8 KB (627 words) - 21:30, 22 November 2023 |
TBST (category Buffer solutions) molecular biology, TBST (or TTBS) is a mixture of tris-buffered saline (TBS) (a buffer solution) and Polysorbate 20 (a polysorbate-type nonionic surfactant)... 1 KB (163 words) - 22:15, 2 March 2024 |
Tris-buffered saline (TBS) is a buffer used in some biochemical techniques to maintain the pH within a relatively narrow range. Tris (with HCl) has a... 2 KB (224 words) - 22:18, 16 January 2022 |
Acid–base homeostasis (redirect from Body buffer) occurred. But buffers cannot correct abnormal pH levels in a solution, be that solution in a test tube or in the extracellular fluid. Buffers typically consist... 21 KB (2,421 words) - 14:25, 19 February 2024 |
One use of conjugate acids and bases lies in buffering systems, which include a buffer solution. In a buffer, a weak acid and its conjugate base (in the... 13 KB (1,298 words) - 00:59, 6 February 2024 |
 | Ringer's solution is a solution of several salts dissolved in water for the purpose of creating an isotonic solution relative to the body fluids of an... 6 KB (613 words) - 06:49, 18 October 2022 |
 | The bicarbonate buffer system is an acid-base homeostatic mechanism involving the balance of carbonic acid (H2CO3), bicarbonate ion (HCO− 3), and carbon... 14 KB (1,731 words) - 08:49, 26 April 2024 |
chemistry, a knowledge of pKa values is necessary for the preparation of buffer solutions and is also a prerequisite for a quantitative understanding of the... 103 KB (11,511 words) - 11:25, 28 April 2024 |
