COVID-19 drug development is the research process to develop preventative therapeutic prescription drugs that would alleviate the severity of coronavirus...
90 KB (13,124 words) - 04:28, 26 July 2024
safe drugs with repurposing potential against COVID-19. Drug repurposing usually requires three steps before taking the drug across the development pipeline:...
128 KB (14,816 words) - 01:34, 9 September 2024
Nirmatrelvir/ritonavir (redirect from Pfizer COVID-19 pill)
United States Food and Drug Administration (FDA) granted nirmatrelvir/ritonavir emergency use authorization (EUA) to treat COVID‑19. It was approved in the...
67 KB (6,032 words) - 10:06, 8 September 2024
therefore was haram or forbidden for purposes of Islamic law. COVID‑19 drug development COVID‑19 drug repurposing research "(OWID) vaccination maps". Our World...
200 KB (22,796 words) - 05:34, 12 September 2024
Lianhua Qingwen (category COVID-19 drug development)
ingredient used to make the drug methamphetamine. Despite the ban, Lianhua Qingwen has been sold illegally in Australia as a COVID-19 treatment. Liang, Xinlu...
16 KB (1,667 words) - 09:45, 15 August 2024
The Pfizer–BioNTech COVID-19 vaccine, sold under the brand name Comirnaty, is an mRNA-based COVID-19 vaccine developed by the German biotechnology company...
260 KB (22,082 words) - 05:55, 13 September 2024
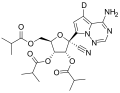
Nirmatrelvir (category COVID-19 drug development)
could be replaced by a rigid pyrrolidone. These drugs had been further developed prior to the COVID-19 pandemic for other diseases including SARS. The...
25 KB (2,010 words) - 16:27, 30 August 2024
Pemivibart (category COVID-19 drug development)
authorized for the pre-exposure prophylaxis (prevention) of COVID‑19. The US Food and Drug Administration (FDA) issued an emergency use authorization for...
7 KB (399 words) - 02:33, 16 September 2024
for COVID-19. Neither drug has been useful to prevent or treat SARS-CoV-2 infection. Administration of chloroquine or hydroxychloroquine to COVID-19 patients...
59 KB (5,538 words) - 20:50, 13 September 2024
Molnupiravir (category COVID-19 drug development)
potential COVID-19 drug". Science. doi:10.1126/science.abc7055. Cully M (January 2022). "A tale of two antiviral targets - and the COVID-19 drugs that bind...
54 KB (4,223 words) - 21:58, 7 September 2024
Drug development is the process of bringing a new pharmaceutical drug to the market once a lead compound has been identified through the process of drug...
26 KB (2,861 words) - 11:27, 24 July 2024
prepare for an outbreak and hasten the development of a preventive COVID-19 vaccine. Since 2020, vaccine development has been expedited via unprecedented...
122 KB (11,033 words) - 22:07, 17 July 2024
developing a therapy against COVID-19 was progressing at a pandemic speed, yet there is no effective therapy approved by food and drug administration (FDA)....
55 KB (5,313 words) - 19:42, 12 September 2024
Baricitinib (category COVID-19 drug development)
treating people with COVID-19. The drug's anti-inflammatory activity was expected to act on the inflammatory cascade associated with COVID-19. In April and June...
43 KB (3,613 words) - 02:13, 16 July 2024
Remdesivir (category COVID-19 drug development)
Technology to Enable the Formulation of Remdesivir in Treating COVID-19". Drug Development & Delivery. Archived from the original on 20 July 2020. Retrieved...
113 KB (9,807 words) - 01:49, 16 July 2024
Deuremidevir (category COVID-19 drug development)
duration of COVID-19 symptoms in non-hospitalized adults with mild-to-moderate disease compared to placebo. Junshi, which markets the drug, received conditional...
5 KB (481 words) - 07:29, 14 August 2024
Simnotrelvir/ritonavir (category COVID-19 drug development)
Xiannuoxin) is a pharmaceutical drug used for the treatment of COVID-19. Simnotrelvir/ritonavir is a combination drug of simnotrelvir, an inhibitor of...
8 KB (334 words) - 16:03, 9 August 2024
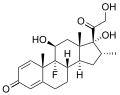
Dexamethasone (category COVID-19 drug development)
acknowledged. "EMA to review results from study testing common steroid drug against COVID-19". Reuters. 24 July 2020. Archived from the original on 27 July 2020...
68 KB (5,999 words) - 20:41, 31 August 2024
Ensitrelvir (category COVID-19 drug development)
Japan to accelerate the progression to market of other antiviral drugs targeting COVID-19, including remdesivir and molnupiravir. In a study of 428 patients...
17 KB (1,228 words) - 07:01, 9 September 2024
Favipiravir (category COVID-19 drug development)
of mild to moderate COVID-19 in Egypt, Hungary and Serbia. Patients are required to sign a consent form before obtaining the drug.[citation needed] Favipiravir...
35 KB (3,139 words) - 21:09, 12 August 2024
Levilimab (category COVID-19 drug development)
approved as a treatment for COVID-19 in Russia. Harrison, Charlotte (1 August 2020). "Focus shifts to antibody cocktails for COVID-19 cytokine storm". Nature...
4 KB (234 words) - 17:45, 25 April 2024
president of Madagascar, claims can prevent and cure Coronavirus disease 2019 (COVID-19). The drink is produced from a species under the Artemisia genus from which...
16 KB (1,462 words) - 08:15, 29 June 2024
and Drug Administration (FDA). Retrieved December 1, 2020. "Coronavirus (COVID-19) Update: FDA Authorizes Drug Combination for Treatment of COVID-19". US...
174 KB (19,757 words) - 01:07, 18 September 2024
MK-7845 (category COVID-19 drug development)
experimental antiviral medication being studied as a potential treatment for COVID-19. It is believed to work by inhibiting SARS-CoV-2 main protease (3CLpro)...
7 KB (581 words) - 12:55, 9 September 2024
Casirivimab/imdevimab (redirect from Regeneron COVID treatment)
after testing positive for COVID‑19. In January 2021, the United States agreed to purchase 1.25 million doses of the drug for $2.625 billion, at $2,100...
49 KB (3,403 words) - 03:31, 7 September 2024
Bamlanivimab/etesevimab (category COVID-19 drug development)
2022. "FDA Authorizes Monoclonal Antibodies for Treatment of COVID-19". U.S. Food and Drug Administration (FDA) (Press release). 10 February 2021. Retrieved...
16 KB (1,301 words) - 07:13, 18 July 2024
The Janssen COVID‑19 vaccine, sold under the brand name Jcovden, is a COVID‑19 vaccine that was developed by Janssen Vaccines in Leiden, Netherlands, and...
141 KB (11,328 words) - 07:32, 10 August 2024
Tipiracil (category COVID-19 drug development)
Tipiracil is a drug used in the treatment of cancer. It is approved for use in form of the combination drug trifluridine/tipiracil for the treatment of...
7 KB (567 words) - 07:53, 16 November 2023
The Novavax COVID-19 vaccine, sold under the brand names Nuvaxovid and Covovax, among others, is a subunit COVID-19 vaccine developed by Novavax and the...
73 KB (5,473 words) - 01:56, 17 September 2024
Vilobelimab (category COVID-19 drug development)
inflammation and worsening of COVID-19. Vilobelimab was granted an emergency use authorization (EUA) by the US Food and Drug Administration (FDA) in April...
8 KB (440 words) - 06:30, 28 December 2023