| Pfizer–BioNTech COVID-19 vaccine (redirect from Comirnaty) The Pfizer–BioNTech COVID-19 vaccine, sold under the brand name Comirnaty, is an mRNA-based COVID-19 vaccine developed by the German biotechnology company... 236 KB (19,394 words) - 05:58, 20 April 2024 |
is the first RNAi drug to receive regulatory approval. The second is Comirnaty, the COVID-19 mRNA vaccine developed by Pfizer/BioNTech that has received... 19 KB (1,222 words) - 09:34, 28 April 2024 |
name Comirnaty, is an mRNA vaccine produced by the German company BioNTech and the American company Pfizer. In Hong Kong, Macau, and Taiwan, Comirnaty is... 318 KB (19,351 words) - 20:00, 21 April 2024 |
Pfizer–BioNTech COVID-19 vaccine codenamed BNT162b2 (later branded as Comirnaty). This approval enabled the start of the UK's COVID-19 vaccination programme... 24 KB (2,284 words) - 11:01, 6 March 2024 |
vaccines, of which the first authorized were COVID-19 vaccines (such as Comirnaty and Spikevax). mRNA is produced by synthesising a ribonucleic acid (RNA)... 24 KB (2,503 words) - 11:29, 22 February 2024 |
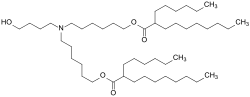 | Erstens stimmt es nicht, dass die Zusatzstoffe unerlaubt sind. Denn Comirnaty ist zugelassen und damit sind auch alle darin enthaltenen Hilfsstoffe... 8 KB (782 words) - 12:58, 24 January 2024 |
$2 billion NxStage acquisition". Modern Healthcare. "Package Insert - Comirnaty". U.S. Food and Drug Administration. December 2021. Retrieved 2022-05-24... 19 KB (1,639 words) - 08:00, 18 April 2024 |
for Pfizer and BioNTech's COVID-19 vaccine, Comirnaty, and its nonproprietary name, tozinameran. Comirnaty was the first COVID-19 vaccine brand name approved... 10 KB (885 words) - 15:16, 23 April 2024 |
 | Today. 2020;S1359-6446(20)30509-2. doi:10.1016/j.drudis.2020.11.025 "Comirnaty and Pfizer-BioNTech COVID-19 Vaccine". FDA. January 3, 2022. Archived... 116 KB (11,587 words) - 06:13, 27 April 2024 |
December 2020. "EMA recommends standard marketing authorizations for Comirnaty and Spikevax COVID-19 vaccines". European Medicines Agency. 16 September... 192 KB (22,021 words) - 19:29, 15 April 2024 |
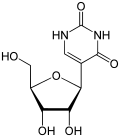 | vaccine from BioNTech/Pfizer, also known as BNT162b2, tozinameran or Comirnaty, all U's have been substituted with N1-methylpseudouridine, a nucleoside... 24 KB (2,806 words) - 06:49, 1 April 2024 |
from Pfizer". ICU Medical. Retrieved 28 April 2017. "Package Insert - Comirnaty". U.S. Food and Drug Administration. December 2021. Retrieved 2022-05-24... 17 KB (1,388 words) - 23:45, 26 October 2023 |
injection" (PDF). Regulation 174. MHRA. 15 December 2020. Assessment report, Comirnaty, Common name: COVID-19 mRNA vaccine (nucleoside-modified); Procedure No... 2 KB (205 words) - 21:16, 8 February 2024 |
provisionally approved the two-dose Pfizer–BioNTech COVID-19 vaccine, named COMIRNATY, for use within Australia. The provisional approval only recommends the... 23 KB (1,797 words) - 14:29, 7 January 2024 |
 | president Donald Trump to emphasize that the pandemic started in China. Comirnaty The commercial name for the FDA approved COVID-19 vaccine from Pfizer... 8 KB (852 words) - 09:41, 11 January 2024 |