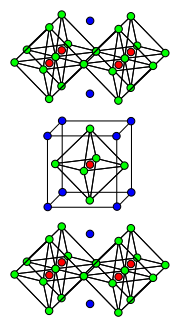
Copper(I) oxide or cuprous oxide is the inorganic compound with the formula Cu2O. It is one of the principal oxides of copper, the other being copper(II)...
13 KB (1,108 words) - 16:07, 30 April 2024
well characterized. Copper oxide may refer to: Copper(I) oxide (cuprous oxide, Cu2O) Copper(II) oxide (cupric oxide, CuO) Copper peroxide (CuO2), a hypothetical...
2 KB (221 words) - 10:59, 8 April 2024
or copper(I) oxide (cuprous oxide). As a mineral, it is known as tenorite. It is a product of copper mining and the precursor to many other copper-containing...
10 KB (856 words) - 14:47, 13 January 2024
Black oxide or blackening is a conversion coating for ferrous materials, stainless steel, copper and copper based alloys, zinc, powdered metals, and silver...
10 KB (1,236 words) - 03:46, 28 April 2024
red-orange color. Copper also has a range of different organic and inorganic salts, having varying oxidation states ranging from (0,I) to (III). These...
8 KB (101 words) - 01:46, 14 November 2023
Copper(I) sulfide is a copper sulfide, a chemical compound of copper and sulfur. It has the chemical compound Cu2S. It is found in nature as the mineral...
6 KB (569 words) - 19:24, 1 April 2024
Copper forms a rich variety of compounds, usually with oxidation states +1 and +2, which are often called cuprous and cupric, respectively. Copper compounds...
14 KB (1,485 words) - 06:57, 13 May 2024
Benedict's reagent (category Copper compounds)
reagent produces a cuprous (Cu+), which precipitates as insoluble red copper(I) oxide (Cu2O). The test is named after American chemist Stanley Rossiter Benedict...
8 KB (912 words) - 11:35, 1 May 2024
element. One exception is copper, for which the highest oxidation state oxide is copper(II) oxide and not copper(I) oxide. Another exception is fluoride...
12 KB (1,600 words) - 20:57, 8 December 2023
presence of monosaccharides. It is based on the reduction of copper(II) acetate to copper(I) oxide (Cu2O), which forms a brick-red precipitate. RCHO + 2Cu2+...
3 KB (293 words) - 10:02, 6 April 2024
the formation of copper(I) oxide (Cu2O). The Cu2O compound has versatile applications such as for use in solar cells, for the oxidation of fiberglass, and...
10 KB (1,169 words) - 03:23, 21 July 2023
solid produced by heating an intimate mixture of barium oxide, copper(II) oxide, and lanthanum oxide in the presence of oxygen. The material was discovered...
3 KB (272 words) - 00:51, 21 July 2023
Sodium oxide, which reacts with water to produce sodium hydroxide Magnesium oxide, which reacts with hydrochloric acid to form magnesium chloride Copper(II)...
5 KB (573 words) - 14:39, 7 December 2023
industrially by treating copper metal with hot concentrated sulfuric acid or copper oxides with dilute sulfuric acid. For laboratory use, copper sulfate is usually...
28 KB (2,433 words) - 10:30, 16 May 2024
Cuprite (redirect from Red copper ore)
Cuprite is an oxide mineral composed of copper(I) oxide Cu2O, and is a minor ore of copper. Its dark crystals with red internal reflections are in the...
5 KB (467 words) - 04:42, 29 April 2024
Yttrium barium copper oxide (YBCO) is a family of crystalline chemical compounds that display high-temperature superconductivity; it includes the first...
23 KB (2,489 words) - 12:12, 21 April 2024
tetrahedite-tennantite, and enargite, copper carbonates such as azurite and malachite, and as copper(I) or copper(II) oxides such as cuprite and tenorite, respectively...
120 KB (13,743 words) - 16:47, 13 May 2024
sodium-containing impurities. Furthermore, this form of copper hydroxide tends to convert to black copper(II) oxide: Cu(OH)2 → CuO + H2O A purer product can be attained...
14 KB (1,338 words) - 06:37, 23 December 2023
Thermite (section Copper thermite)
bismuth(III) oxide, boron(III) oxide, silicon(IV) oxide, chromium(III) oxide, manganese(IV) oxide, iron(III) oxide, iron(II,III) oxide, copper(II) oxide, and...
49 KB (5,766 words) - 19:39, 5 May 2024
Copper oxide selenite is an inorganic compound with the chemical formula Cu2OSeO3. It is an electrically insulating, piezoelectric and piezomagnetic material...
6 KB (548 words) - 18:21, 17 May 2024
experimental results on various aspects have been gained in the case of copper(I) oxide. Oscillations in ionized gases were observed by Lewi Tonks and Irving...
11 KB (1,204 words) - 20:43, 10 January 2024
Glass-to-metal seal (section Copper)
metal-oxide interface. Proper thickness of the oxide layer is therefore critical. Metallic copper does not bond well to glass. Copper(I) oxide, however...
35 KB (4,950 words) - 12:59, 20 November 2023
Han purple and Han blue (redirect from Barium Copper Silicate)
form copper (I) oxide is 3 BaCuSi2O6 → BaCuSi4O10 + 2 BaSiO3 + 2 CuO Above 1050 °C, the CuO copper (II) oxide breaks down to copper (I) oxide: 4 CuO → 2...
25 KB (2,953 words) - 13:04, 12 March 2024
native vermilion (the common ore of mercury). Copper Glance – copper(I) sulfide ore. Cuprite – copper(I) oxide ore. Dutch White – a pigment, formed from one...
14 KB (1,523 words) - 17:16, 23 January 2024
Bismuth strontium calcium copper oxide (BSCCO, pronounced bisko), is a type of cuprate superconductor having the generalized chemical formula Bi2Sr2Can−1CunO2n+4+x...
14 KB (1,582 words) - 10:06, 22 February 2024
List of inorganic compounds (section I)
– CoF3 Copper(I) acetylide – Cu2C2 Copper(I) chloride – CuCl Copper(I) fluoride – CuF Copper(I) oxide – Cu2O Copper(I) sulfate – CuSO4 Copper(I) sulfide...
119 KB (8,726 words) - 04:34, 18 April 2024
become discolored due to the facile aerobic oxidation of the iodide anion to molecular iodine. Copper(I) iodide, like most binary (containing only two...
12 KB (1,120 words) - 02:37, 13 January 2024
Reducing sugar (section Oxidation-reduction)
reducing sugar reduces the copper(II) ions in these test solutions to copper(I), which then forms a brick red copper(I) oxide precipitate. Reducing sugars...
14 KB (1,690 words) - 12:56, 15 March 2024
List of fungicides (section I)
copper(II) acetate copper(II) carbonate, basic copper hydroxide copper naphthenate copper oleate [Wikidata] copper(I) oxide copper oxychloride copper(II)...
17 KB (1,109 words) - 19:03, 13 May 2024
The 1:2 ratio of copper and oxygen would be consistent with copper in its common +2 oxidation state and a peroxide group. Although samples of this composition...
4 KB (317 words) - 02:03, 1 April 2024