| Sulfur monoxide (section Disulfur dioxide) dilute gas phase. When concentrated or condensed, it converts to S2O2 (disulfur dioxide). It has been detected in space but is rarely encountered intact otherwise... 14 KB (1,400 words) - 02:12, 9 February 2024 |
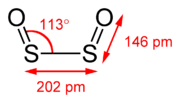
simply be SO, as is the empirical formula of disulfur dioxide, S2O2. Thus, sulfur monoxide and disulfur dioxide, both compounds of sulfur and oxygen, have... 5 KB (730 words) - 09:13, 19 April 2024 |
dimer, Disulfur dioxide (S2O2) Sulfur dioxide (SO2) Sulfur trioxide (SO3) Higher sulfur oxides (SO3 and SO4 and polymeric condensates of them) Disulfur monoxide... 829 bytes (102 words) - 17:18, 12 October 2023 |
 | Sulfur dioxide (IUPAC-recommended spelling) or sulphur dioxide (traditional Commonwealth English) is the chemical compound with the formula SO 2. It is... 52 KB (7,281 words) - 20:52, 24 April 2024 |
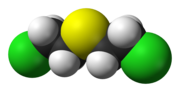
 | Disulfur dichloride (or disulphur dichloride by the British English spelling) is the inorganic compound of sulfur and chlorine with the formula S2Cl2.... 9 KB (723 words) - 15:44, 21 December 2023 |
 | match smell similar to sulfur dioxide. Disulfur decafluoride is produced by photolysis of SF5Br: 2 SF5Br → S2F10 + Br2 Disulfur decafluoride arises by the... 8 KB (646 words) - 18:15, 18 March 2023 |
mononitride, SN, analogous to nitric oxide, NO disulfur mononitride, S2N, analogous to nitrogen dioxide, NO2. monosulfur dinitride, SN2, analogous to nitrous... 955 bytes (115 words) - 08:22, 7 November 2022 |
In organometallic chemistry, metal sulfur dioxide complexes are complexes that contain sulfur dioxide, SO2, bonded to a transition metal. Such compounds... 8 KB (908 words) - 02:15, 15 January 2023 |
 | Disulfur difluoride is an inorganic compound with the chemical formula S2F2. It is a halide of sulfur. Disulfur difluoride has a chain structure F−S−S−F... 4 KB (382 words) - 20:20, 4 February 2024 |
including the sulfur-rich oxides include sulfur monoxide, disulfur monoxide, disulfur dioxides, and higher oxides containing peroxo groups. Sulfur reacts... 10 KB (1,150 words) - 04:23, 9 February 2024 |
the ratio x:y is greater than 1:2 Disulfur monoxide, S2O Disulfur dioxide, S2O2 Sulfur monoxide, SO Sulfur dioxide, SO2 Sulfur trioxide, SO3 Higher sulfur... 8 KB (601 words) - 06:38, 4 April 2024 |
 | acids. SCl2 is produced by the chlorination of either elemental sulfur or disulfur dichloride. The process occurs in a series of steps, some of which are:... 6 KB (432 words) - 22:19, 30 April 2023 |
 | Ozone (redirect from Oxygen dioxide) 25% that of carbon dioxide. The annual global warming potential of tropospheric ozone is between 918 and 1022 tons carbon dioxide equivalent/tons tropospheric... 148 KB (18,046 words) - 02:51, 11 April 2024 |
 | reacts with sulfur to form disulfur dibromide) S2Br2 + 8 H2O + 5 Br2 → 2 H2SO4 + 12 HBr (oxidation and hydration of disulfur dibromide) Prior to 1900,... 65 KB (7,200 words) - 05:24, 17 April 2024 |
 | Silylone (section Carbon dioxide) Lewis basic properties despite not being a Si(0) complex. For example, the disulfur complex can then form an adduct with GaCl3. XRD analysis of this GaCl3-coordinated... 24 KB (2,895 words) - 18:18, 22 September 2023 |
perchlorate) – ClOClO3 Dichlorine trioxide – Cl2O3 Dichlorosilane – SiH2Cl2 Disulfur dichloride – S2Cl2 Dysprosium(III) chloride – DyCl3 Erbium(III) chloride... 119 KB (8,726 words) - 04:34, 18 April 2024 |
 | analogue Disulfur dinitride Bis(trimethylsilyl)sulfur diimide Kresze, G.; Wucherpfennig, W., "Organic synthesis with imides of sulfur dioxide", Angew.... 3 KB (366 words) - 15:17, 23 June 2023 |
 | Allotropes of sulfur (section Disulfur, S2) consist of criss-crossed helices. It is also called χ-sulfur or ω2-sulfur. Disulfur, S2, is the predominant species in sulfur vapour above 720 °C (a temperature... 26 KB (2,475 words) - 10:55, 15 February 2024 |
 | is a low oxidation state (+1) sulfur acid. It is the Arrhenius acid for disulfur monoxide. Salts derived from thiosulfurous acid, which are also unknown... 6 KB (666 words) - 11:55, 17 October 2023 |
features in the UV: SO2, FeCl3, Cl2, Sn, SCl2, S2O, elemental sulfur, and disulfur dioxide (S 2O 2). It has also been speculated that any hypothetical microorganisms... 13 KB (923 words) - 17:08, 31 January 2022 |
Diazodinitrophenol Diazomethane Diethyl ether peroxide 4-Dimethylaminophenylpentazole Disulfur dinitride Ethyl azide Explosive antimony Fluorine perchlorate Fulminic... 73 KB (8,326 words) - 08:56, 25 March 2024 |
Among the best known toxic gases are carbon monoxide, chlorine, nitrogen dioxide and phosgene. Toxic: a chemical that has a median lethal concentration... 14 KB (584 words) - 10:45, 21 April 2024 |
by 400 °C. Up to 800° it loses some sulfur dioxide. From 800° to 850 °C it loses sulfur dioxide and disulfur resulting in cerium oxy disulfate, and dioxy... 17 KB (1,520 words) - 11:53, 22 September 2022 |