 | The European Medicines Agency (EMA) is an agency of the European Union (EU) in charge of the evaluation and supervision of pharmaceutical products. Prior... 43 KB (3,701 words) - 10:53, 29 April 2024 |
ensuring that medicines and medical devices work and are acceptably safe. The MHRA was formed in 2003 with the merger of the Medicines Control Agency (MCA) and... 24 KB (2,284 words) - 11:01, 6 March 2024 |
responsible for the regulatory activity of pharmaceuticals in Italy. European Medicines Agency Istituto Superiore di Sanità Official website v t e v t e... 464 bytes (37 words) - 18:24, 13 May 2024 |
 | © European Medicines Agency. Reproduction is authorized provided the source is acknowledged. "Dapagliflozin Viatris EPAR". European Medicines Agency. 4... 53 KB (4,937 words) - 05:04, 12 May 2024 |
Biosimilar (section European Union) their lifecycle. Drug-related authorities such as the European Medicines Agency (EMA) of the European Union, the United States Food and Drug Administration... 95 KB (6,479 words) - 21:14, 31 March 2024 |
 | European Medicines Agency in the European Union. In January 2018, the Committee for Medicinal Products for Human Use of the European Medicines Agency... 17 KB (1,585 words) - 19:15, 28 April 2024 |
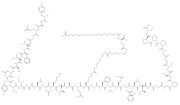 | the brand name Zepbound. In November 2023, the UK Medicines and Healthcare products Regulatory Agency revised the indication for tirzepatide to include... 42 KB (3,387 words) - 15:37, 9 May 2024 |
 | N-Nitrosodimethylamine (section European Union) is © European Medicines Agency. Reproduction is authorized provided the source is acknowledged. "Nitrosamine impurities". European Medicines Agency (EMA)... 25 KB (2,390 words) - 04:57, 9 May 2024 |
 | and methods of analysis for medicines. These standards apply to medicines for both human and veterinary use. The European Pharmacopoeia has a legally... 11 KB (1,225 words) - 06:47, 24 April 2024 |
Committee for Medicinal Products for Human Use (CHMP) of the European Medicines Agency (EMA) adopted a positive opinion, recommending the granting of... 20 KB (1,433 words) - 07:08, 3 March 2024 |
 | medicines-cancelled-tga-and-recalled-pharmacies-safety-reasons "EMA recommends withdrawal of pholcodine medicines from EU market". European Medicines... 17 KB (1,566 words) - 16:24, 15 February 2024 |
 | The European Directorate for the Quality of Medicines & HealthCare (EDQM) is a Directorate and partial agreement of the Council of Europe that traces... 33 KB (3,770 words) - 16:29, 27 April 2024 |
2023, the Committee for Medicinal Products for Human Use of the European Medicines Agency (EMA) recommended a conditional marketing authorization for epcoritamab... 13 KB (821 words) - 04:46, 9 May 2024 |
 | vaccine has since been approved by several medicine agencies worldwide, such as the European Medicines Agency (EMA), and the Australian Therapeutic Goods... 213 KB (16,972 words) - 00:44, 9 May 2024 |
Adalimumab (category World Health Organization essential medicines) EPAR". European Medicines Agency (EMA). 14 September 2021. Archived from the original on 24 April 2022. Retrieved 23 April 2022. "Hulio EPAR". European Medicines... 91 KB (6,940 words) - 04:46, 30 April 2024 |
copyright European Medicines Agency. Reproduction is authorized provided the source is acknowledged. "Pyzchiva EPAR". European Medicines Agency. February... 25 KB (1,922 words) - 02:45, 30 April 2024 |
 | population as opposed to Spaniards. An assessment report by the European Medicines Agency remarked that "the potential to induce agranulocytosis may be... 53 KB (4,614 words) - 04:59, 3 May 2024 |
the European Medicines Agency in January 2023. On 20 July 2023, the Committee for Medicinal Products for Human Use (CHMP) of the European Medicines Agency... 13 KB (1,033 words) - 03:46, 8 February 2024 |
approved by the health agency of the country like FDA (Food and Drug Administration) for USA, or EMA (European Medicines Agency) for Europe. Regulatory professionals... 9 KB (1,059 words) - 11:56, 16 February 2024 |
 | Committee for Medicinal Products for Human Use (CHMP) of the European Medicines Agency (EMA) adopted a positive opinion, recommending the granting of... 14 KB (804 words) - 03:51, 31 March 2024 |
Bevacizumab (category World Health Organization essential medicines) which is © European Medicines Agency. Reproduction is authorized provided the source is acknowledged. "Aybintio EPAR". European Medicines Agency (EMA). 26... 79 KB (7,144 words) - 06:09, 17 April 2024 |
 | Committee for Medicinal Products for Human Use (CHMP) of the European Medicines Agency (EMA) adopted a positive opinion, recommending the granting of... 25 KB (1,916 words) - 21:37, 20 December 2023 |
 | which is © European Medicines Agency. Reproduction is authorized provided the source is acknowledged. "Opzelura EPAR". European Medicines Agency. 20 April... 26 KB (2,090 words) - 02:10, 2 April 2024 |







