Fluorine (9F) has 18 known isotopes ranging from 13 F to 31 F (with the exception of 30 F ) and two isomers (18m F and 26m F ). Only fluorine-19 is stable... 13 KB (856 words) - 16:43, 16 August 2023 |
 | Halogen (redirect from Fluorine family) group in the periodic table consisting of six chemically related elements: fluorine (F), chlorine (Cl), bromine (Br), iodine (I), and the radioactive elements... 52 KB (5,485 words) - 16:34, 28 April 2024 |
 | Hydrogen fluoride (redirect from Fluorine hydride) solution termed hydrofluoric acid. It is the principal industrial source of fluorine, often in the form of hydrofluoric acid, and is an important feedstock... 15 KB (1,366 words) - 01:19, 9 March 2024 |
Fluorine forms a great variety of chemical compounds, within which it always adopts an oxidation state of −1. With other atoms, fluorine forms either polar... 85 KB (8,549 words) - 12:04, 15 March 2024 |
 | Fluorine-18 (18F) is a fluorine radioisotope which is an important source of positrons. It has a mass of 18.0009380(6) u and its half-life is 109.771(20)... 8 KB (872 words) - 14:54, 6 February 2024 |
 | Fluorine perchlorate, also called perchloryl hypofluorite is the rarely encountered chemical compound of fluorine, chlorine, and oxygen with the chemical... 5 KB (505 words) - 06:38, 24 July 2023 |
FKM (redirect from Fluorine rubber) ISO standard 1629. It is commonly called fluorine rubber or fluoro-rubber. FKM is an abbreviation of Fluorine Kautschuk Material. All FKMs contain vinylidene... 13 KB (1,421 words) - 15:07, 17 April 2024 |
 | Fluorine is a relatively new element in human applications. In ancient times, only minor uses of fluorine-containing minerals existed. The industrial... 21 KB (2,322 words) - 02:33, 31 March 2024 |
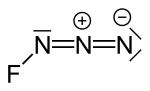 | Fluorine azide or triazadienyl fluoride is a yellow green gas composed of nitrogen and fluorine with formula FN3. Its properties resemble those of ClN3... 8 KB (724 words) - 12:54, 11 January 2024 |
 | Fluorocarbon (section Electrochemical fluorination) Fluorocarbons are chemical compounds with carbon-fluorine bonds. Compounds that contain many C-F bonds often have distinctive properties, e.g., enhanced[clarification... 25 KB (2,364 words) - 17:15, 24 March 2024 |
 | The fluorine cycle is the series of biogeochemical processes through which fluorine moves through the lithosphere, hydrosphere, atmosphere, and biosphere... 8 KB (842 words) - 13:06, 14 March 2024 |
Halogenation (redirect from Fluorination) Fluorine and chlorine are more electrophilic and are more aggressive halogenating agents. Bromine is a weaker halogenating agent than both fluorine and... 10 KB (1,113 words) - 19:34, 28 April 2024 |
Fluorine absorption dating is a method used to determine the amount of time an object has been underground. Fluorine absorption dating is based on the... 2 KB (243 words) - 04:40, 25 February 2023 |
Chlorine fluoride (redirect from Fluorine chloride) chlorine fluoride is an interhalogen compound containing only chlorine and fluorine. National Pollutant Inventory - Fluoride compounds fact sheet NIST Standard... 2 KB (24 words) - 13:25, 5 August 2017 |
 | Fluorodeoxyglucose (18F) (redirect from Fluorine-18 fluorodeoxyglucose) a glucose analog, with the positron-emitting radionuclide fluorine-18 substituted for the normal hydroxyl group at the C-2 position in the... 22 KB (2,441 words) - 09:15, 19 April 2024 |
Radical fluorination is a type of fluorination reaction, complementary to nucleophilic and electrophilic approaches. It involves the reaction of an independently... 21 KB (2,126 words) - 01:06, 29 January 2023 |
 | Fluorite (category Fluorine minerals) hydrofluoric acid manufacture, which is the intermediate source of most fluorine-containing fine chemicals. Optically clear transparent fluorite has anomalous... 34 KB (3,748 words) - 22:06, 19 April 2024 |
Dioxygen monofluoride (redirect from Fluorine superoxide) Dioxygen monofluoride is a binary inorganic compound radical of fluorine and oxygen with the chemical formula O2F. The compound is stable only at low temperature... 4 KB (313 words) - 08:43, 11 September 2023 |
Period 2 element (section Fluorine) contains the elements lithium, beryllium, boron, carbon, nitrogen, oxygen, fluorine, and neon. In a quantum mechanical description of atomic structure, this... 35 KB (3,902 words) - 16:25, 20 April 2024 |
Electrochemical fluorination (ECF), or electrofluorination, is a foundational organofluorine chemistry method for the preparation of fluorocarbon-based... 5 KB (669 words) - 00:03, 11 March 2024 |
chemistry of organofluorine compounds, organic compounds that contain a carbon–fluorine bond. Organofluorine compounds find diverse applications ranging from oil... 42 KB (4,757 words) - 23:58, 16 April 2024 |
Fluorination with aminosulfuranes is a chemical reaction that transforms oxidized organic compounds into organofluorine compounds. Aminosulfuranes selectively... 11 KB (1,111 words) - 21:33, 21 December 2023 |
Dioxygen difluoride (redirect from Fluorine dioxide) Dioxygen difluoride is a compound of fluorine and oxygen with the molecular formula O2F2. It can exist as an orange-colored solid which melts into a red... 11 KB (1,061 words) - 04:53, 13 August 2023 |






