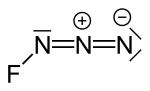Fluorine azide or triazadienyl fluoride is a yellow green gas composed of nitrogen and fluorine with formula FN3. Its properties resemble those of ClN3...
8 KB (724 words) - 12:54, 11 January 2024
dissociate at room temperature and above to give the radical NF2•. Fluorine azide (FN3) is very explosive and thermally unstable. Dinitrogen difluoride...
105 KB (12,188 words) - 15:49, 18 April 2024
first identified in 1952 as the thermal decomposition product of the fluorine azide (FN3). It has the structure F−N=N−F and exists in both cis and trans...
7 KB (574 words) - 04:35, 14 February 2024
decomposition. Azide decomposition offers a less-efficient but more pure technique: fluorine azide (which can be formed in situ via reaction of atomic fluorine with...
4 KB (352 words) - 03:01, 9 April 2024
Bromine azide is an explosive inorganic compound with the formula BrN3. It has been described as a crystal or a red liquid at room temperature.[citation...
6 KB (523 words) - 14:43, 13 December 2023
azide (IN3) is an explosive inorganic compound, which in ordinary conditions is a yellow solid. Formally, it is an inter-pseudohalogen. Iodine azide can...
7 KB (579 words) - 14:45, 13 December 2023
dissociate at room temperature and above to give the radical NF2•. Fluorine azide (FN3) is very explosive and thermally unstable. Dinitrogen difluoride...
34 KB (4,297 words) - 09:04, 25 February 2024
pentafluoride, NF5 Dinitrogen difluoride, N2F2 Tetrafluorohydrazine, N2F4 Fluorine azide, N3F Tetrafluoroammonium, NF4+ This set index article lists chemical...
656 bytes (73 words) - 18:30, 7 June 2023
azide is prepared by passing chlorine gas over silver azide, or by an addition of acetic acid to a solution of sodium hypochlorite and sodium azide....
4 KB (262 words) - 00:31, 4 December 2023
dinitride Ethyl azide Explosive antimony Fluorine perchlorate Fulminic acid Halogen azides: Fluorine azide Chlorine azide Bromine azide Iodine azide Hexamethylene...
73 KB (8,329 words) - 06:43, 9 May 2024
hydroperoxide and fluorine in the presence of cesium fluoride". Inorganic Chemistry. 11 (1): 193–195. doi:10.1021/ic50107a047. Journal of Fluorine Chemistry....
94 KB (4,456 words) - 10:02, 12 May 2024
Halogenation (redirect from Fluorination)
Fluorine and chlorine are more electrophilic and are more aggressive halogenating agents. Bromine is a weaker halogenating agent than both fluorine and...
10 KB (1,113 words) - 19:34, 28 April 2024
other than fluorines, such as benzene rings, are also allowed on the cyclooctyne. This reaction has been used successfully to probe for azides in living...
48 KB (5,477 words) - 03:30, 5 April 2024
dipole moments can be high: cyanamide 4.27 D, diazomethane 1.5 D, methyl azide 2.17, pyridine 2.19. For this reason many compounds containing CN bonds...
8 KB (677 words) - 13:33, 22 August 2023
atomic number 17. The second-lightest of the halogens, it appears between fluorine and bromine in the periodic table and its properties are mostly intermediate...
116 KB (13,006 words) - 19:00, 16 May 2024
to a specific halogenoalkane. Haloalkanes containing carbon bonded to fluorine, chlorine, bromine, and iodine results in organofluorine, organochlorine...
20 KB (2,394 words) - 13:06, 14 April 2024
groups such as fluorine increase rate by decreasing LUMO energy and the HOMO-LUMO gap. This leads to a greater charge transfer from the azide to the fluorinated...
44 KB (5,098 words) - 05:29, 19 February 2024
17 of the periodic table. Its properties are thus similar to those of fluorine, chlorine, and iodine, and tend to be intermediate between those of the...
66 KB (7,600 words) - 16:56, 8 May 2024
The azo and azide groups respectively, connected to organic/inorganic compounds (e.g. silver azide AgN3, lead azide Pb(N3)2, ammonium azide NH4N3) III...
3 KB (423 words) - 07:35, 27 January 2023
ligation with organic azides, copper-catalyzed Huisgen cycloaddition of azides, and strain promoted Huisgen cycloaddition of azides. The nucleophilic lysine...
43 KB (4,661 words) - 07:44, 6 March 2024
Lithium azide is the lithium salt of hydrazoic acid. It is an unstable and toxic compound that decomposes into lithium and nitrogen when heated. It can...
3 KB (136 words) - 13:14, 2 December 2023
compound is a molecule which contains two or more different halogen atoms (fluorine, chlorine, bromine, iodine, or astatine) and no atoms of elements from...
20 KB (2,291 words) - 15:33, 22 January 2024
hemoglobin and certain cytochromes in a manner analogous to cyanide and azide. The two principal sulfur oxides are obtained by burning sulfur: S + O2...
10 KB (1,150 words) - 04:23, 9 February 2024
carbon-fluorine bonds in perfluoroalkanes, which are usually unreactive due to fluorine’s high electronegativity and the strength of the carbon-fluorine bond...
15 KB (1,486 words) - 07:19, 6 May 2024
fourth halogen, being a member of group 17 in the periodic table, below fluorine, chlorine, and bromine; it is the heaviest stable member of its group....
106 KB (11,803 words) - 00:50, 15 May 2024
hydrochloride Silane, (4-aminobutyl)diethoxymethyl- Sodium arsenate Sodium azide Sodium cacodylate Sodium cyanide Sodium fluoroacetate Sodium pentachlorophenate...
10 KB (825 words) - 05:28, 10 December 2023
dangerously explosive silver compounds are silver azide, AgN3, formed by reaction of silver nitrate with sodium azide, and silver acetylide, Ag2C2, formed when...
94 KB (11,249 words) - 22:16, 10 April 2024
having partially filled octets and therefore acting as Lewis acids. note 1 Fluorine is too electronegative to be bonded to magnesium; it becomes an ionic salt...
31 KB (1,192 words) - 12:11, 6 March 2024
room temperature. Some examples of which are solid nitrogen, triazane, azide anion and triazoles. Even longer series with eight nitrogen atoms or more...
12 KB (1,465 words) - 04:12, 15 April 2024
Pb2+ cations. Borderline bases are: aniline, pyridine, nitrogen N2 and the azide, chloride, bromide, nitrate and sulfate anions. Generally speaking, acids...
19 KB (2,114 words) - 23:19, 15 December 2023