The Food and Drug Administration is a federal agency of the United States, formed in 1930. Up until the 20th century, there were few federal laws regulating... 26 KB (3,396 words) - 13:22, 5 May 2024 |
Numerous governmental and non-governmental organizations have criticized the U. S. Food and Drug Administration for alleged excessive and/or insufficient regulation... 46 KB (5,595 words) - 07:02, 6 May 2024 |
 | giving authority to the U.S. Food and Drug Administration (FDA) to oversee the safety of food, drugs, medical devices, and cosmetics. The FDA's principal... 33 KB (3,338 words) - 06:37, 29 March 2024 |
 | Food and Drug Administration is a ministry of the Government of Maharashtra. The ministry is responsible for consumer protection and regulating food and... 17 KB (333 words) - 11:39, 4 May 2024 |
 | Nootropic (redirect from Nootropic Drug) Center for Food Safety and Applied Nutrition, Inspections, Compliance, Enforcement, and Criminal Investigations, US Food and Drug Administration. Retrieved... 32 KB (3,182 words) - 04:15, 30 April 2024 |
The Department of Food and Drug Administration (Burmese: အစားအသောက်နှင့် ဆေးဝါးကွပ်ကဲရေး ဦးစီးဌာန; abbreviated FDA) is Burma's food safety regulatory... 2 KB (123 words) - 01:01, 30 March 2023 |
 | The Food and Drug Administration Revitalization Act was introduced by the 101st Congress of the United States. Senator Orrin G. Hatch was the chairperson... 13 KB (844 words) - 18:53, 21 April 2024 |
Anticonvulsant (redirect from Anticonvulsant drug) Drugs@FDA. U.S. Food and Drug Administration (FDA). Retrieved 21 November 2019. "New Drug Application (NDA) 013263". Drugs@FDA. U.S. Food and Drug Administration... 60 KB (5,294 words) - 22:47, 7 April 2024 |
Food and Drug Administration, Maharashtra State, is Maharashtra's primary instrument of consumer protection. It is a law enforcement agency. In 1970, the... 1 KB (63 words) - 14:23, 8 November 2023 |
 | of China Food and Drug Administration (FDA; Chinese: 食品藥物管理署) is a Republic of China government agency, which is responsible for the safety and quality... 5 KB (290 words) - 01:52, 24 October 2023 |
and Drug Administration. 30 January 2018. Retrieved 18 April 2024. "FDA - Germany, MOU on Good Laboratory Practice". U.S. Food and Drug Administration. 31... 36 KB (3,629 words) - 20:27, 5 May 2024 |
Food and Drug Administration may refer to: China Food and Drug Administration (NMPA) Food and Drug Administration, a government agency in the United States... 392 bytes (80 words) - 22:19, 20 December 2019 |
FDA v. Alliance for Hippocratic Medicine (redirect from Alliance for Hippocratic Medicine v. US Food and Drug Administration) Food and Drug Administration v. Alliance for Hippocratic Medicine is a pending United States Supreme Court case to challenge the U.S. Food and Drug Administration... 45 KB (4,676 words) - 16:19, 20 April 2024 |
 | Dietary supplement (redirect from Food supplements) the label must bear a disclaimer that the Food and Drug Administration (FDA) "has not evaluated the claim" and that the dietary supplement product is not... 100 KB (11,061 words) - 09:10, 21 April 2024 |
 | The Drug Enforcement Administration (DEA) is a United States federal law enforcement agency under the U.S. Department of Justice tasked with combating... 83 KB (8,347 words) - 14:44, 10 April 2024 |
 | The United States Food and Drug Administration's Investigational New Drug (IND) program is the means by which a pharmaceutical company obtains permission... 7 KB (846 words) - 08:52, 21 March 2024 |
 | Semaglutide (category Drugs with non-standard legal status) original weight within 5 years of stopping treatment. The US Food and Drug Administration (FDA) approved semaglutide based on evidence from seven clinical... 66 KB (5,371 words) - 20:10, 1 May 2024 |
the United States Food and Drug Administration that regulates food, dietary supplements, and cosmetics, The Food and Drug Administration (United States)... 9 KB (1,088 words) - 18:28, 20 April 2024 |
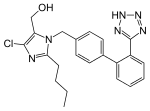 | Losartan (category Drugs with non-standard legal status) October 2014, the U.S. Food and Drug Administration (FDA) issued a black box warning that losartan can cause fetal toxicity, and should be discontinued... 41 KB (3,253 words) - 17:30, 7 February 2024 |
modes of action, and/or are used to treat the similar diseases. The Food and Drug Administration (FDA) has worked on classifying and licensing new medications... 10 KB (1,018 words) - 11:15, 1 May 2024 |









