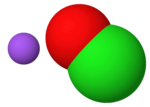
| Hypochlorous acid is an inorganic compound with the chemical formula ClOH, also written as HClO, HOCl, or ClHO. Its structure is H−O−Cl. It is an acid... 48 KB (5,422 words) - 10:24, 20 March 2024 |
hypohalous acid is an oxyacid consisting of a hydroxyl group single-bonded to any halogen. Examples include hypofluorous acid, hypochlorous acid, hypobromous... 4 KB (396 words) - 20:46, 10 May 2022 |
Chlorine acid can refer to: Hydrochloric acid, HCl Hypochlorous acid, HClO Chlorous acid, HClO2 Chloric acid, HClO3 Perchloric acid, HClO4 Chlorine acids Molecular... 940 bytes (81 words) - 03:41, 29 December 2023 |
 | Disinfectant (section Peroxy and peroxo acids) Trichloroisocyanuric acid Chlorine dioxide Hypochlorous acid Iodine Iodophors Sodium hydroxide Potassium hydroxide Calcium hydroxide Magnesium hydroxide Sulfurous acid Sulfur... 44 KB (4,903 words) - 07:45, 20 April 2024 |
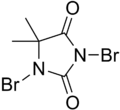 | involve the use of hypochlorous acid. 1,3-Dibromo-5,5-Dimethylhydantoin is a source of bromine, which is equivalent to hypobromous acid (HOBr). Br2X + 2... 4 KB (201 words) - 15:12, 1 June 2023 |
 | Hypochlorite (section Acid reaction) of hypochlorites generates hypochlorous acid, which exists in an equilibrium with chlorine. A lowered pH (ie. towards acid) drives the following reaction... 18 KB (1,725 words) - 18:08, 16 January 2024 |
 | Chlorine (redirect from Dephlogisticated marine acid) Chlorine forms four oxoacids: hypochlorous acid (HOCl), chlorous acid (HOClO), chloric acid (HOClO2), and perchloric acid (HOClO3). As can be seen from... 113 KB (12,719 words) - 19:41, 20 March 2024 |
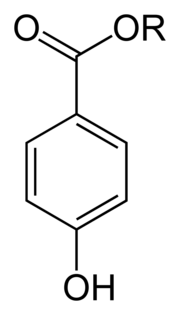 | Paraben (section 4-Hydroxybenzoic acid (PHBA)) spectroscopy has confirmed that chlorine is predominantly present as hypochlorous acid (HClO). Parabens can react with HClO to form mono- and di- chlorinated... 32 KB (3,973 words) - 16:20, 10 January 2024 |
of dissolved salt to produce chlorine gas or its dissolved forms, hypochlorous acid and sodium hypochlorite, which are already commonly used as sanitizing... 10 KB (1,329 words) - 23:32, 24 March 2024 |
medium (pH 8.5–11). The acting chlorinating agent in this reaction is hypochlorous acid (HOCl), which has to be generated by protonation of hypochlorite,... 20 KB (2,037 words) - 04:15, 15 April 2024 |
 | the hypochlorous acid that was formed from the initial BCDMH: Br− + HOCl → HOBr + Cl− This produces more hypobromous acid; the hypochlorous acid itself... 5 KB (301 words) - 22:21, 4 February 2024 |
 | The conjugate base is known in salts such as lithium hypofluorite. Hypochlorous acid, a related compound that is more technologically important but has... 6 KB (493 words) - 00:21, 13 February 2024 |
Oxyacid (redirect from Oxygen acid) chlorine has the four following oxyacids: hypochlorous acid HClO chlorous acid HClO2 chloric acid HClO3 perchloric acid HClO4 Some elemental atoms can exist... 22 KB (1,761 words) - 06:52, 2 May 2024 |
by acidifying with hydrochloric acid. It can also be made by passing acetylene through solutions of hypochlorous acid.[citation needed] As a laboratory... 21 KB (1,910 words) - 20:05, 24 April 2024 |
 | Dichlorine monoxide (redirect from Hypochlorous anhydride) chlorine oxide family of compounds, as well as being the anhydride of hypochlorous acid. It is a strong oxidiser and chlorinating agent. The earliest method... 14 KB (1,319 words) - 15:13, 13 January 2024 |
to form hypochlorous acid, a very weak acid: Cl2O + H2O ⇌ 2 HOCl Chlorine(VII) oxide reacts with water to form perchloric acid, a strong acid: Cl2O7 +... 5 KB (580 words) - 02:09, 29 April 2024 |
 | Denaturation (biochemistry) (redirect from Nucleic acid denaturation) peroxide, elemental chlorine, hypochlorous acid (chlorine water), bromine, bromine water, iodine, nitric and oxidising acids, and ozone react with sensitive... 31 KB (3,056 words) - 07:11, 15 April 2024 |
chloride ions, myeloperoxidase uses hydrogen peroxide to produce hypochlorous acid. H2O2 + Cl− —> ClO− + H2O Nitric oxide synthase (the inducible isoform... 22 KB (2,555 words) - 10:14, 5 November 2023 |
Acid strength is the tendency of an acid, symbolised by the chemical formula HA {\displaystyle {\ce {HA}}} , to dissociate into a proton, H + {\displaystyle... 18 KB (2,564 words) - 21:07, 20 February 2024 |
to cleave glycols, α-hydroxy carboxylic acids and keto acids to yield fragmented aldehydes or carboxylic acids. Calcium hypochlorite can also be used in... 9 KB (822 words) - 07:10, 2 April 2024 |