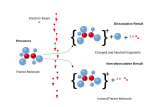
| three ionization energies are defined as follows: 1st ionization energy is the energy that enables the reaction X ⟶ X+ + e− 2nd ionization energy is the... 51 KB (5,883 words) - 03:07, 9 March 2024 |
a singly, doubly, etc., charged ion. For ionization energies measured in the unit eV, see Ionization energies of the elements (data page). All data from... 25 KB (229 words) - 06:23, 13 November 2023 |
 | ionization rate is possible. Tunnel ionization is ionization due to quantum tunneling. In classical ionization, an electron must have enough energy to... 42 KB (6,098 words) - 19:23, 5 April 2024 |
column marked 1 is the first ionization energy to ionize the neutral atom, the column marked 2 is the second ionization energy to remove a second electron... 63 KB (870 words) - 17:41, 8 January 2024 |
 | Periodic trends (section Ionization energy) the nucleus. It is also referred to as ionization potential. The first ionization energy is the amount of energy that is required to remove the first electron... 15 KB (1,607 words) - 14:03, 15 March 2024 |
 | Ion (section Ionization potential) electric charge is called the ionization potential, or ionization energy. The nth ionization energy of an atom is the energy required to detach its nth electron... 30 KB (3,020 words) - 15:27, 7 April 2024 |
therefore the bulk of the ionization effects are due to secondary ionization. Even though photons are electrically neutral, they can ionize atoms indirectly through... 60 KB (6,536 words) - 14:05, 21 April 2024 |
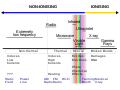
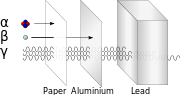 | Radiation (section Ionizing radiation) enough heat to raise temperatures to ionization energies. These reactions occur at far higher energies than with ionization radiation, which requires only single... 47 KB (6,126 words) - 20:17, 11 April 2024 |
Bond energy and bond-dissociation energy Gravitational binding energy Ionization energy (binding energy of one electron) Nuclear binding energy Quantum... 14 KB (1,389 words) - 20:37, 12 March 2024 |
 | Stopping power (particle radiation) (redirect from Non-ionizing energy loss) electron) requires a fixed amount of energy (for example, 33.97 eV in dry air: 305 ), the number of ionizations per path length is proportional to the... 28 KB (3,729 words) - 09:09, 4 November 2023 |
In physics, the Saha ionization equation is an expression that relates the ionization state of a gas in thermal equilibrium to the temperature and pressure... 12 KB (1,824 words) - 15:48, 19 January 2024 |
process are ionized. Thermal ionization is used to make simple ion sources, for mass spectrometry and for generating ion beams. Thermal ionization has seen... 5 KB (652 words) - 00:40, 1 September 2023 |
 | Ion source (redirect from Atmospheric pressure ionization) Electron ionization is widely used in mass spectrometry, particularly for organic molecules. The gas phase reaction producing electron ionization is M +... 58 KB (7,140 words) - 08:46, 21 February 2024 |
first two ionization energies for europium, 1632 kJ·mol−1 can be compared with that of barium 1468.1 kJ·mol−1 and europium's third ionization energy is the... 106 KB (10,220 words) - 14:34, 21 April 2024 |
which approximately corresponds to both the first ionization energy of oxygen, and the ionization energy of hydrogen at about 14 eV. When photons come into... 13 KB (1,486 words) - 07:22, 27 September 2023 |
 | Mass spectrometry (redirect from Soft ionization) example of hard ionization is electron ionization (EI). Soft ionization refers to the processes which impart little residual energy onto the subject... 82 KB (10,342 words) - 00:29, 17 April 2024 |
electronegativity of an atom is strongly correlated with the first ionization energy. The electronegativity is slightly negatively correlated (for smaller... 36 KB (4,507 words) - 20:46, 28 April 2024 |
but this generally requires low ionization energies. Instead, because of its small size and high ionization energies, the basic structural unit of boron... 249 KB (28,114 words) - 15:21, 1 April 2024 |
Enthalpy of solution Heat of dilution Hydrate Hydrational fluid Ionization energy Greenwood, Norman N.; Earnshaw, Alan (1997). Chemistry of the Elements... 3 KB (345 words) - 16:35, 28 September 2023 |
 | Plasma (physics) (redirect from Ionized gas) a plasma. The degree of plasma ionization is determined by the electron temperature relative to the ionization energy (and more weakly by the density)... 62 KB (6,399 words) - 10:58, 15 April 2024 |
orbital approximation). Ionization energies calculated this way are in qualitative agreement with experiment – the first ionization energy of small molecules... 24 KB (2,875 words) - 05:33, 25 October 2023 |
Hence, its first ionization energy would be smaller than thorium's (Th: 6.3 eV; element 122: 5.6 eV) because of the greater ease of ionizing unbibium's 8p1/2... 148 KB (15,104 words) - 12:51, 19 April 2024 |
core level to final states in the energy region of 50–100 eV above the selected atomic core level ionization energy, where the wavelength of the photoelectron... 15 KB (1,958 words) - 03:45, 19 March 2023 |
 | the estimated value for the s2p configuration. In 2015, the first ionization energy of lawrencium was measured, using the isotope 256Lr. The measured... 40 KB (8,059 words) - 12:54, 4 February 2024 |
 | Paschen's law (section Impact ionization) which can be accelerated to trigger impact ionization. Such so-called seed electrons can be created by ionization by natural radioactivity or cosmic rays... 22 KB (2,785 words) - 20:40, 13 February 2024 |
Rydberg state (section Energy of Rydberg states) electronically excited states with energies that follow the Rydberg formula as they converge on an ionic state with an ionization energy. Although the Rydberg formula... 6 KB (653 words) - 14:29, 1 December 2023 |
 | highly ionized regions (H II regions and the Warm Ionised Medium). Carbon has a lower ionization energy than hydrogen, and so singly-ionized carbon atoms... 51 KB (5,769 words) - 17:35, 5 April 2024 |