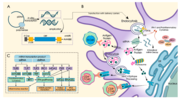
 | Moderna COVID-19 vaccine (redirect from MRNA-1273) mRNA-1273/background/2021.1. Scholia has a profile for mRNA-1273 vaccine (Q87775025). Wikimedia Commons has media related to MRNA-1273. Wikinews... 165 KB (13,017 words) - 17:59, 27 April 2024 |
 | Solid lipid nanoparticle (redirect from MRNA-lipid nanoparticle) a complex of plasmid or linear DNA and lipids Targeted drug delivery mRNA-1273, from Moderna, uses LNPs BNT162b2, from BioNTech/Pfizer, uses LNPs Saupe... 23 KB (2,629 words) - 15:37, 19 March 2024 |
PMID 30785039. Safety and Immunogenicity Study of 2019-nCoV Vaccine (mRNA-1273) for Prophylaxis of SARS-CoV-2 Infection (COVID-19), clinicaltrials.gov... 7 KB (628 words) - 18:17, 27 March 2024 |
 | ; Hernán, Miguel A. (2022-07-01). "Comparative Safety of BNT162b2 and mRNA-1273 Vaccines in a Nationwide Cohort of US Veterans". JAMA Internal Medicine... 16 KB (1,510 words) - 19:37, 11 April 2024 |
COVID-19 vaccine (redirect from MRNA-lipid nanoparticle COVID-19 vaccines) mRNA-1273/background/2021.1. Archived from the original on 13 June 2021. Retrieved 24 July 2021. "Background document on the mRNA-1273 vaccine... 192 KB (21,930 words) - 19:29, 15 April 2024 |
 | the Moderna COVID‑19 vaccine candidate, and in December, the vaccine, mRNA-1273, was issued an emergency use authorization in the United States. In 2022... 23 KB (1,818 words) - 19:04, 19 April 2024 |
Nucleoside-modified messenger RNA (redirect from Nucleoside-modified mRNA) developed by the cooperation of BioNTech/Pfizer (BNT162b2), and by Moderna (mRNA-1273). The zorecimeran vaccine developed by Curevac, however, uses unmodified... 24 KB (2,503 words) - 11:29, 22 February 2024 |
 | Study to Evaluate Safety, Reactogenicity, and Immunogenicity of mRNA-1283 and mRNA-1273 Vaccines in Healthy Adults Between 18 Years and 55 Years of Age... 4 KB (275 words) - 09:58, 30 December 2023 |
 | mRNA-1273/background/2021.1. Archived from the original on 13 June 2021. Retrieved 24 July 2021. "Background document on the mRNA-1273 vaccine... 285 KB (32,672 words) - 14:17, 28 April 2024 |
 | 2020, Moderna struck a manufacturing deal with Lonza to produce its (mRNA-1273) COVID-19 vaccine active ingredient while expanding sterile production... 18 KB (1,817 words) - 19:54, 20 March 2024 |
 | and vote on regulations relating to public health. On 8 January 2021, MRNA-1273 (commonly known as the Moderna COVID-19 vaccine) was the third COVID-19... 181 KB (19,458 words) - 05:24, 20 April 2024 |
 | which biotechnology company Moderna used as the basis for the vaccine mRNA-1273, the first COVID-19 vaccine candidate to enter phase I clinical trials... 30 KB (2,822 words) - 17:59, 15 February 2024 |
advisory panel to consider recommending emergency use authorization of the mRNA-1273 vaccine. Moore is married to Janet Kosloff, a retired CEO and entrepreneur... 11 KB (895 words) - 20:41, 24 October 2023 |
vaccines: AZD1222 from Oxford University and AstraZeneca on 30 December, mRNA-1273 from Moderna on 8 January 2021, and a single-dose vaccine from Janssen... 24 KB (2,284 words) - 11:01, 6 March 2024 |
vaccines approved for use in humans include the RNA vaccines tozinameran and mRNA-1273, the DNA vaccine ZyCoV-D as well as the viral vectors AZD1222, Ad26.COV2... 8 KB (1,186 words) - 19:47, 27 October 2022 |
via public health clinics. The province began to administer the Moderna MRNA-1273 vaccine in early-January 2021, with an initial focus on Northern Saskatchewan... 257 KB (22,391 words) - 20:38, 21 December 2023 |
 | A. Doria-Rose; Xiaoying Shen; et al. (20 December 2021). "Booster of mRNA-1273 Strengthens SARS-CoV-2 Omicron Neutralization". medRxiv 10.1101/2021.12... 297 KB (38,840 words) - 17:09, 25 April 2024 |
Melanie; Miller, Jacqueline; Zaks, Tal (2021). "Efficacy and Safety of the mRNA-1273 SARS-CoV-2 Vaccine". New England Journal of Medicine. 384 (5): 403–416... 5 KB (462 words) - 16:13, 4 May 2023 |
 | to 30,000 volunteers assigned to one of two groups—one receiving the mRNA-1273 vaccine and the other receiving salt water injections—and continued until... 65 KB (6,175 words) - 18:14, 24 April 2024 |
January 2021. "Safety and Immunogenicity Study of 2019-nCoV Vaccine (mRNA-1273) for Prophylaxis of SARS-CoV-2 Infection (COVID-19)". ClinicalTrials.gov... 318 KB (19,351 words) - 20:00, 21 April 2024 |
axicabtagene ciloleucel (Yescarta) is approved. 2020 – The first mRNA vaccines (BNT162b2, mRNA-1273), are developed for SARS-CoV-2 infection; this new technology... 19 KB (2,182 words) - 05:09, 22 December 2023 |