In chemistry, the mass fraction of a substance within a mixture is the ratio w i {\displaystyle w_{i}} (alternatively denoted Y i {\displaystyle Y_{i}}... 5 KB (835 words) - 18:26, 6 March 2024 |
Mass fraction may refer to: Mass fraction (chemistry), it is the ratio of mass of a constituent to the total mass of the mixture Fuel mass fraction Propellant... 421 bytes (96 words) - 05:03, 21 April 2021 |
A fraction in chemistry is a quantity collected from a batch of a substance in a fractionating separation process. In such a process, a mixture is separated... 2 KB (189 words) - 00:21, 3 February 2024 |
In chemistry and fluid mechanics, the volume fraction φ i {\displaystyle \varphi _{i}} is defined as the volume of a constituent Vi divided by the volume... 4 KB (640 words) - 17:33, 14 December 2023 |
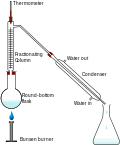 | solubility at a given temperature. In cell fractionation, cell components are separated by difference in mass. A typical protocol to isolate a pure chemical... 4 KB (452 words) - 19:49, 20 April 2024 |
In chemistry, the mole fraction or molar fraction, also called mole proportion or molar proportion, is a quantity defined as the ratio between the amount... 10 KB (1,839 words) - 12:47, 2 September 2023 |
Mass-independent isotope fractionation or Non-mass-dependent fractionation (NMD), refers to any chemical or physical process that acts to separate isotopes... 11 KB (1,220 words) - 08:20, 25 October 2023 |
In polymer chemistry, the molar mass distribution (or molecular weight distribution) describes the relationship between the number of moles of each polymer... 10 KB (1,391 words) - 17:17, 4 February 2024 |
 | Concentration (redirect from Concentration (chemistry)) mole fraction. The SI unit is mol/mol. However, the deprecated parts-per notation is often used to describe small mole ratios. The mass fraction w i {\displaystyle... 10 KB (1,251 words) - 06:40, 18 March 2024 |
The mass-flux fraction (or Hirschfelder-Curtiss variable or Kármán-Penner variable) is the ratio of mass-flux of a particular chemical species to the total... 2 KB (346 words) - 15:46, 3 February 2024 |
 | In chemistry, a solution is a special type of homogeneous mixture composed of two or more substances. In such a mixture, a solute is a substance dissolved... 14 KB (1,883 words) - 18:48, 1 April 2024 |
In chemistry, the mass concentration ρi (or γi) is defined as the mass of a constituent mi divided by the volume of the mixture V. ρ i = m i V {\displaystyle... 7 KB (1,096 words) - 03:51, 16 October 2023 |
mg→mEq:element mass [mg]mass fraction×VMV{\displaystyle {\text{mg}}\to {\text{mEq}}:\quad {\frac {\text{element mass [mg]}}{\text{mass fraction}}}\times {\frac... 8 KB (673 words) - 00:00, 14 April 2024 |
 | Parts-per notation (category Analytical chemistry) in chemistry, for instance, the relative abundance of dissolved minerals or pollutants in water. The quantity "1 ppm" can be used for a mass fraction if... 27 KB (2,393 words) - 05:27, 26 October 2023 |
by mass fraction (in commercial contexts often called weight fraction), by mole fraction (fraction of atoms by numerical count, or sometimes fraction of... 39 KB (4,072 words) - 20:20, 25 April 2024 |
 | In chemistry, the molar mass (M) of a chemical compound is defined as the ratio between the mass and the amount of substance (measured in moles) of any... 18 KB (2,796 words) - 13:58, 24 April 2024 |
Binding energy (redirect from Mass Defect) reactions, the fraction of mass that may be removed as light or heat, i.e. binding energy, is often a much larger fraction of the system mass. It may thus... 14 KB (1,389 words) - 20:37, 12 March 2024 |
In chemistry, the lever rule is a formula used to determine the mole fraction (xi) or the mass fraction (wi) of each phase of a binary equilibrium phase... 6 KB (852 words) - 18:13, 12 May 2023 |
Mixture fraction (Z{\displaystyle Z}) is a quantity used in combustion studies that measures the mass fraction of one stream (usually the fuel stream)... 5 KB (1,008 words) - 12:34, 10 June 2022 |
 | Amount of substance (redirect from Amount (chemistry)) contains 6.02214076×1023 water molecules) has a total mass of about 18.015 grams. In chemistry, because of the law of multiple proportions, it is often... 29 KB (3,601 words) - 03:37, 8 April 2024 |
Molar concentration (section Mass fraction) i {\displaystyle M_{i}} is the molar mass of constituent i {\displaystyle i} . The conversion to mole fraction x i {\displaystyle x_{i}} is given by... 11 KB (1,391 words) - 01:08, 16 April 2024 |
in organic mass spectrometry. Cambridge, Eng: Royal Society of Chemistry. ISBN 0-85404-570-8. JURGEN H. GROSS; Jnrgen H. Gross (2004). Mass Spectrometry:... 7 KB (926 words) - 18:06, 10 December 2020 |
are essential for the study of chemistry; some of them are: In chemistry, matter is defined as anything that has rest mass and volume (it takes up space)... 83 KB (9,163 words) - 13:54, 21 April 2024 |
Mixing ratio (category Dimensionless numbers of chemistry) refer either to mole ratio (see concentration) or mass ratio (see stoichiometry). In atmospheric chemistry, mixing ratio usually refers to the mole ratio... 5 KB (757 words) - 16:55, 3 May 2021 |
Equivalent weight (redirect from Equivalent weight (chemistry)) In chemistry, equivalent weight (also known as gram equivalent or equivalent mass) is the mass of one equivalent, that is the mass of a given substance... 19 KB (2,682 words) - 00:31, 26 April 2024 |
Molality (category Mass-specific quantities) In chemistry, molality is a measure of the amount of solute in a solution relative to a given mass of solvent. This contrasts with the definition of molarity... 21 KB (5,000 words) - 04:51, 6 March 2024 |




