The Medicines and Healthcare products Regulatory Agency (MHRA) is an executive agency of the Department of Health and Social Care in the United Kingdom... 24 KB (2,284 words) - 11:01, 6 March 2024 |
of regulatory agencies that enforce standards include the Food and Drug Administration in the United States and the Medicines and Healthcare products Regulatory... 10 KB (1,040 words) - 22:04, 29 December 2023 |
its promotional materials. Subsequently, the UK Medicines And Healthcare Products Regulatory Agency launched an investigation after it was revealed that... 19 KB (1,538 words) - 06:29, 26 September 2023 |
protection products and fertilizers), energy, banking, telecom etc. Regulatory affairs also has a very specific meaning within the healthcare industries... 9 KB (1,059 words) - 11:56, 16 February 2024 |
Ayurvedic and traditional Chinese medicines Archived 2006-08-30 at the Wayback Machine Medicines and Healthcare products Regulatory Agency Some Ayurvedic... 3 KB (252 words) - 12:05, 19 April 2024 |
 | Ranitidine (category World Health Organization essential medicines) 13 over-the-counter Ranitidine medicines". GOV.uk. United Kingdom: Medicines and Healthcare products Regulatory Agency (MHRA). 21 November 2019. Archived... 99 KB (8,572 words) - 05:06, 31 March 2024 |
 | European Medicines Agency (EMA) is an agency of the European Union (EU) in charge of the evaluation and supervision of pharmaceutical products. Prior to... 38 KB (3,464 words) - 18:14, 27 March 2024 |
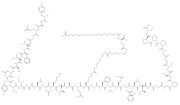 | Tirzepatide (category Drugs developed by Eli Lilly and Company) the brand name Zepbound. In November 2023, the UK Medicines and Healthcare products Regulatory Agency revised the indication for tirzepatide to include... 42 KB (3,387 words) - 04:55, 20 April 2024 |
Nicorette (section Further product) Safety of Medicines Working Group on Nicotine Replacement Therapy, November 2005" (PDF). Medicines and Healthcare products Regulatory Agency. 2005. Archived... 43 KB (3,887 words) - 10:30, 14 April 2024 |
 | Bayer (redirect from Bayer HealthCare LLC) include: pharmaceuticals, consumer healthcare products, agricultural chemicals, seeds and biotechnology products. The company is a component of the EURO... 119 KB (11,418 words) - 15:10, 21 April 2024 |
 | Semaglutide (section Structure and pharmacology) (semaglutide) and Saxenda (liraglutide): vigilance required due to potentially harmful falsified products". Medicines and Healthcare products Regulatory Agency. 23... 66 KB (5,383 words) - 10:23, 22 April 2024 |
South Mimms was merged with the Medicines and Healthcare products Regulatory Agency (MHRA). The Health Protection Agency (HPA) was originally established... 12 KB (1,332 words) - 11:41, 7 December 2023 |
 | United States, the Medicines and Healthcare products Regulatory Agency in the United Kingdom, the Spanish Agency of Medicines and Medical Devices in Spain... 4 KB (353 words) - 18:11, 2 November 2023 |
 | Regulation of therapeutic goods (redirect from Drug regulatory agency) technical advice Medicines for Human Use in the United Kingdom are regulated by the Medicines and Healthcare products Regulatory Agency (MHRA). The availability... 33 KB (3,813 words) - 18:21, 4 March 2024 |
not been approved by the US Food and Drug Administration or the UK Medicines and Healthcare products Regulatory Agency. It is illegal to sell apetamin... 2 KB (195 words) - 05:09, 6 April 2024 |
Chiron Corporation (category 2006 mergers and acquisitions) unsolved until the MHRA (Medicines and Healthcare Products Regulatory Agency), the British equivalent of the FDA stepped in and suspended Chiron's license... 23 KB (2,299 words) - 20:32, 15 April 2024 |
Exagamglogene autotemcel (category Drugs acting on the blood and blood forming organs) estimated to be £1 million. "Summary of Product Characteristics". Medicines and Healthcare products Regulatory Agency (MHRA). 15 November 2023. Archived from... 20 KB (1,433 words) - 07:08, 3 March 2024 |
 | Nirmatrelvir/ritonavir (section Society and culture) 18 January 2022. "Summary of Product Characteristics for Paxlovid". Medicines and Healthcare products Regulatory Agency (MHRA). 31 December 2021. Archived... 64 KB (5,751 words) - 07:52, 24 March 2024 |
on Human Medicines (CHM) is a committee of the UK's Medicines and Healthcare products Regulatory Agency. It was formed in October 2005, and assumed the... 4 KB (527 words) - 10:29, 2 February 2024 |
 | Esophagitis (section Signs and symptoms) Medical Concept Library. Retrieved 22 July 2021. Medicines and Healthcare Products Regulatory Agency (MHRA), 2013. Metoclopramide: risk of neurological... 15 KB (1,788 words) - 13:36, 29 February 2024 |
Sciensus (redirect from Healthcare at Home) suspension" of its licence by the Medicines and Healthcare products Regulatory Agency (MHRA). In 2013 Healthcare at Home took over almost 3,000 patients from... 12 KB (829 words) - 20:28, 24 October 2023 |
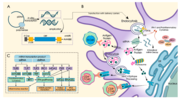 | MRNA vaccine (section Polymer and peptide vectors) final eight-week trial, the UK Medicines and Healthcare products Regulatory Agency (MHRA) became the first global medicines regulator in history to approve... 77 KB (7,847 words) - 09:09, 18 March 2024 |
 | review of the matter by the Medicines and Healthcare products Regulatory Agency in 2014 assessed the studies performed to date, and concluded that it found... 8 KB (688 words) - 19:40, 1 March 2024 |
 | Molnupiravir (section Society and culture) Retrieved 16 August 2023. "Summary of Product Characteristics for Lagevrio". Medicines and Healthcare products Regulatory Agency (MHRA). 4 November 2021. Archived... 53 KB (4,209 words) - 15:49, 11 March 2024 |
 | Pfizer–BioNTech COVID-19 vaccine (category Products introduced in 2020) 2020. "UK medicines regulator gives approval for first UK COVID-19 vaccine" (Press release). Medicines and Healthcare products Regulatory Agency (MHRA).... 236 KB (19,394 words) - 05:58, 20 April 2024 |
authorization) of medicines, including vaccines and biologics, which have already been approved by SRAs. As of 2022, the national regulatory authorities of... 6 KB (289 words) - 15:43, 18 March 2024 |




