Molar concentration (also called molarity, amount concentration or substance concentration) is a measure of the concentration of a chemical species, in... 11 KB (1,391 words) - 01:08, 16 April 2024 |
 | be distinguished: mass concentration, molar concentration, number concentration, and volume concentration. The concentration can refer to any kind of... 10 KB (1,251 words) - 06:40, 18 March 2024 |
The molar conductivity of an electrolyte solution is defined as its conductivity divided by its molar concentration. Λ m = κ c , {\displaystyle \Lambda... 11 KB (1,376 words) - 17:32, 28 March 2024 |
 | Amount of substance (redirect from Molar quantity) with the normal isotopic mix is 40.078(4) g/mol. The molar concentration, also called molarity, of a solution of some substance is the number of moles... 29 KB (3,601 words) - 03:37, 8 April 2024 |
magnitude of molar concentration. Source values are parenthesized where unit conversions were performed. M denotes the non-SI unit molar: 1 M = 1 mol/L... 14 KB (814 words) - 15:37, 12 March 2024 |
Molality (section Molar concentration (molarity)) mixed solvent. The term molality is formed in analogy to molarity which is the molar concentration of a solution. The earliest known use of the intensive... 21 KB (5,000 words) - 04:51, 6 March 2024 |
Mole fraction (redirect from Molar fraction) In chemistry, the mole fraction or molar fraction, also called mole proportion or molar proportion, is a quantity defined as the ratio between the amount... 10 KB (1,839 words) - 12:47, 2 September 2023 |
and the concentration of the species, according to the Beer–Lambert law A = ε c ℓ , {\displaystyle A=\varepsilon c\ell ,} where ε is the molar absorption... 6 KB (754 words) - 05:31, 9 September 2023 |
Mass fraction (chemistry) (section Molar concentration) }}.} The relation to molar concentration is like that from above substituting the relation between mass and molar concentration: w i = ρ i ρ = c i M i... 5 KB (835 words) - 18:26, 6 March 2024 |
conversion to molar concentration ci is given by: c i = ρ i M i {\displaystyle c_{i}={\frac {\rho _{i}}{M_{i}}}} where Mi is the molar mass of constituent... 7 KB (1,096 words) - 03:51, 16 October 2023 |
 | For example: glucose has n of 1, while NaCl has n of 2; C is the molar concentration of the solute; the index i represents the identity of a particular... 9 KB (1,206 words) - 03:12, 16 October 2023 |
Reference ranges for blood tests (redirect from Serum concentration) substance concentrations from the molar to the mass concentration scale above are made as follows: Numerically: molar concentration × molar mass = mass... 108 KB (6,105 words) - 18:14, 12 April 2024 |
Number density (redirect from Number concentration) sometimes called concentration, although usually concentration is expressed as a number of moles per unit volume (and thus called molar concentration). For any... 7 KB (809 words) - 06:17, 22 April 2024 |
 | to 360g/L), or between 4.81 and 5.58 mmol/L. It is thus a mass or molar concentration. Still, many instances measure MCHC in percentage (%), as if it were... 5 KB (596 words) - 16:00, 15 January 2024 |
Molar (unit), a unit of concentration equal to 1 mole per litre Molar quantities, such as molar mass, molar volume, etc. El Molar, Tarragona, a village... 690 bytes (131 words) - 05:26, 9 September 2023 |
 | PH (redirect from Hydrogen-ion concentration) {H+}}})\thickapprox -\log([{\ce {H+}}])} where [H+] is the equilibrium molar concentration (mol/L) of H+ in the solution. At 25 °C (77°F), solutions with a... 49 KB (6,168 words) - 07:10, 30 April 2024 |
In chemistry, the equivalent concentration or normality (N) of a solution is defined as the molar concentration ci divided by an equivalence factor or... 5 KB (595 words) - 03:11, 16 October 2023 |
 | In chemistry, the molar mass (M) of a chemical compound is defined as the ratio between the mass and the amount of substance (measured in moles) of any... 18 KB (2,796 words) - 02:42, 1 May 2024 |
Thermodynamic activity (redirect from Effective concentration) the gas phase), molality bi, mass fraction wi, molar concentration (molarity) ci or mass concentration ρi: a i x = γ x , i x i , a i b = γ b , i b i... 21 KB (2,982 words) - 18:11, 21 April 2024 |
 | IC50 (redirect from Half maximal inhibitory concentration) receptor or microorganism. IC50 values are typically expressed as molar concentration. IC50 is commonly used as a measure of antagonist drug potency in... 10 KB (1,072 words) - 13:38, 26 March 2024 |
per mass density Molar extinction coefficient, how strongly a substance absorbs light at a given wavelength, per molar concentration Optical extinction... 756 bytes (116 words) - 20:48, 5 March 2023 |
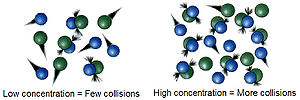 | kg). NA is the Avogadro constant. [A] is molar concentration of A in unit mol⋅L−1. [B] is molar concentration of B in unit mol⋅L−1. Z can be converted... 22 KB (3,223 words) - 18:12, 7 March 2024 |
depends solely on the ratio of the molar concentrations of the weak acid to the weak base. The higher the concentration of the weak acid in the solution... 21 KB (2,421 words) - 14:25, 19 February 2024 |
 | that the product of molar concentration of calcium ions (moles of dissolved Ca2+ per liter of solution) with the molar concentration of dissolved CO2−3... 78 KB (7,435 words) - 19:01, 31 March 2024 |
behavior (P=CRuT), the molar concentration C will remain constant since the pressure and temperature are constant. Therefore, the molar flow rates of each... 6 KB (907 words) - 13:35, 6 March 2022 |
solvent, it is equal to the ratio of its molar concentration in the stationary phase to its molar concentration in the mobile phase, also approximating... 3 KB (362 words) - 01:12, 13 August 2023 |
In polymer chemistry, the molar mass distribution (or molecular weight distribution) describes the relationship between the number of moles of each polymer... 10 KB (1,391 words) - 17:17, 4 February 2024 |


