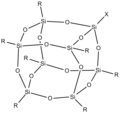
Oppenauer oxidation, named after Rupert Viktor Oppenauer [de], is a gentle method for selectively oxidizing secondary alcohols to ketones. The reaction...
12 KB (1,250 words) - 17:28, 13 May 2022
isopropoxide is used in MPV reductions of ketones and aldehydes and the Oppenauer oxidation of secondary alcohols. In these reactions, it is assumed that the...
9 KB (812 words) - 09:40, 11 April 2024
Alcohol oxidation is a collection of oxidation reactions in organic chemistry that convert alcohols to aldehydes, ketones, carboxylic acids, and esters...
14 KB (1,578 words) - 01:04, 20 April 2024
oxidation Sulfonium-based oxidation of alcohols to aldehydes Pyridinium chlorochromate Jones oxidation Oppenauer oxidation Pfitzner–Moffatt oxidation...
9 KB (1,019 words) - 17:16, 26 December 2023
Dess–Martin periodinane (redirect from Periodic acid oxidation)
reactions. Alcohol oxidation Pyridinium chlorochromate Jones oxidation Oppenauer oxidation Pfitzner–Moffatt oxidation Parikh–Doering oxidation Albright-Goldman...
12 KB (1,197 words) - 15:45, 11 April 2024
reduction. This method, however, has the ability to undergo the reverse Oppenauer oxidation due to the proximity of the two reagents. Thus the reaction runs...
14 KB (1,512 words) - 00:33, 18 April 2024
hydrogenation Olefin isomerization Olefin oxidation Olefin polymerization Oppenauer oxidation Oxidation Oxidative addition Oxidative decarbonylation Oxygenation Oxymercuration...
4 KB (296 words) - 16:47, 16 February 2024
reagent Olefin metathesis Oppenauer oxidation Ostromyslenskii reaction, Ostromisslenskii reaction Overman rearrangement Oxidative decarboxylation Oxo synthesis...
38 KB (3,433 words) - 17:07, 5 January 2024
to the Oppenauer oxidation, which favors the oxidation of secondary alcohols over primary and several other specialty oxidations. Over-oxidation of primary...
20 KB (2,216 words) - 10:06, 7 December 2023
to give α-hydroxy ketones Meerwein–Ponndorf–Verley reduction and Oppenauer oxidation - related interconversions of ketones and secondary alcohols via...
8 KB (930 words) - 18:27, 27 October 2023
to the dioxirane using oxone. It serves as an oxidizing agent in Oppenauer oxidation. Trifluoracetone is also used in a synthesis of 2-trifluoromethyl-7-azaindoles...
5 KB (360 words) - 00:36, 2 May 2024
equation and, for example, the Meerwein–Ponndorf–Verley reduction/Oppenauer oxidation equilibrium for the measurement of axial versus equatorial values...
19 KB (2,380 words) - 14:57, 13 May 2022
reaction Cannizzaro reaction Meerwein–Ponndorf–Verley reduction Oppenauer oxidation Seki, Tsunetake; Nakajo, Tetsuo; Onaka, Makoto (2006). "The Tishchenko...
7 KB (664 words) - 19:00, 3 February 2024
to the corresponding ketone). Selective oxidation of alcohols to aldehydes requires circumventing over-oxidation to the carboxylic acid. One popular approach...
6 KB (729 words) - 16:41, 12 June 2023
Nucleophilic epoxidation Oppenauer oxidation Prilezhaev reaction Rubottom oxidation Schmidt reaction Swern oxidation Wacker-Tsuji oxidation Reduction Birch reduction...
8 KB (576 words) - 12:14, 30 October 2023
hydrogenation), followed by oxidation with benzophenone in presence of potassium tert butoxide or aluminium tert butoxide (Oppenauer oxidation). The 6 ketone group...
43 KB (3,963 words) - 05:59, 4 March 2024
heteroaromatic substrates tend to fail. Meerwein–Ponndorf–Verley reduction Oppenauer oxidation Dehydrogenation Hydrogenation Hydrogenolysis Borrowing hydrogen Wang...
13 KB (1,292 words) - 14:45, 29 February 2024
as also occurs for the first step in the synthesis of nandrolone. Oppenauer oxidation then transforms the C17β hydroxyl group into a ketone functionality...
91 KB (9,305 words) - 06:23, 9 February 2024
of enones, as well as other Lewis acid-catalyzed reactions like Oppenauer oxidation and Meerwein-Pondorf-Verley reductions. . A number of metallasilsesquioxanes...
34 KB (3,746 words) - 22:41, 27 March 2024
produced chiral 28 and on Oppenauer oxidation chiral 29. Hydrogenation (Adams' catalyst) gave alcohol 30, chromic acid oxidation gave ketone 31, sodium borohydride...
9 KB (1,098 words) - 12:52, 14 April 2023
Aldehyde (redirect from Corey-Gilman-Ganem oxidation)
cheap oxygen is the oxidant of choice. For sensitive substrates, Oppenauer transfer oxidation avoids overoxidation to a carboxylic acid. When a mixture of...
29 KB (3,000 words) - 10:16, 11 May 2024
Firstly, the double bond in ring D is hydrogenated, followed by Oppenauer oxidation of the hydroxyl group and the concurrent migration of the remaining...
17 KB (1,828 words) - 22:56, 1 March 2024
Subsequent acetylation with acetic anhydride and tosyl acid followed by Oppenauer oxidation afforded 17a-acetoxy- progesterone (95) in good yield (115). Tests...
26 KB (3,337 words) - 00:05, 9 December 2023
ring (10). The product is saponified and then the subject of an Oppenauer oxidation, which is then dehydrogenated to the 4,6-diene with chloranil (11)...
5 KB (473 words) - 21:31, 8 December 2023
Library. Retrieved October 22, 2017. Arrich, Jasmin; Schütz, Nikola; Oppenauer, Julia; Vendt, Janne; Holzer, Michael; Havel, Christof; Herkner, Harald...
48 KB (5,627 words) - 15:24, 11 May 2024