| The Peterson olefination (also called the Peterson reaction) is the chemical reaction of α-silyl carbanions (1 in diagram below) with ketones (or aldehydes)... 7 KB (727 words) - 03:28, 9 March 2024 |
The Julia olefination (also known as the Julia–Lythgoe olefination) is the chemical reaction used in organic chemistry of phenyl sulfones (1) with aldehydes... 12 KB (1,302 words) - 00:29, 11 February 2023 |
reagent or the Corey–Chaykovsky reagent or α-silyl carbanions in the Peterson olefination a phosphonate carbanion in the Horner–Wadsworth–Emmons reaction a... 8 KB (910 words) - 11:22, 17 April 2024 |
Wittig reaction (redirect from Wittig olefination) reaction Julia olefination Peterson olefination Tebbe's reagent Organophosphorus chemistry Homologation reaction Kauffmann olefination Titanium–zinc methylenation... 15 KB (1,600 words) - 05:31, 18 March 2024 |
 | Tebbe's reagent (redirect from Tebbe olefination) Lombardo reagent McMurry reaction Nysted reagent Peterson olefination Wittig reaction Kauffmann olefination F. N. Tebbe, G. W. Parshall and G. S. Reddy (1978)... 10 KB (907 words) - 21:15, 3 August 2023 |
the sulfur–carbon bonds of sulfones are typically reductive in nature. Olefination or replacement with hydrogen may be accomplished using reductive desulfonylation... 20 KB (2,248 words) - 12:28, 15 January 2024 |
rearrangement Perkow reaction Petasis reaction Petasis reagent Peterson olefination Peterson reaction Petrenko-Kritschenko piperidone synthesis Pfau–Plattner... 38 KB (3,433 words) - 17:07, 5 January 2024 |
Horner–Wadsworth–Emmons reaction (category Olefination reactions) reaction Michaelis–Arbuzov reaction Michaelis–Becker reaction Peterson reaction Tebbe olefination Wadsworth, W. Org. React. 1977, 25, 73. doi:10.1002/0471264180... 9 KB (963 words) - 11:26, 24 January 2024 |
 | oxidizing the less hindered secondary alcohol(scheme 15). Corey-Winter olefination is a stereospecific transformation of 1,2-diols to alkenes involving... 55 KB (5,646 words) - 14:23, 10 May 2024 |
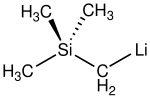 | reaction of (trimethylsilyl)methyl chloride. In one example of the Peterson olefination, (trimethylsilyl)methyllithium reacts with aldehydes and ketones... 7 KB (565 words) - 07:05, 15 May 2024 |
intermediates. These intermediates then undergo intramolecular heteroatom Peterson olefination to yield indolinines, which then tautomerize to 2-substituted indoles... 11 KB (1,278 words) - 00:35, 22 May 2023 |
 | the lithium salt of Trimethyl(phenylthiomethyl)silane 1.16 in a Peterson olefination to the sulfide 1.17 followed by deprotection to completed ring A... 11 KB (1,349 words) - 23:30, 19 June 2023 |
Hoon; Ko, Sangwon; Chang, Sukbok (2004). "Ruthenium-Catalyzed Heck-Type Olefination and Suzuki Coupling Reactions: Studies on the Nature of Catalytic Species"... 34 KB (3,851 words) - 03:55, 15 February 2024 |
thioether) ligands have been use in olefination of free carboxylic acids and arylation, carbonylation, and olefination of free aliphatic amines. Expanding... 25 KB (2,764 words) - 06:39, 17 November 2023 |