 | Pfizer–BioNTech COVID-19 vaccine (redirect from Tozinameran) version of the bivalent vaccine (Comirnaty Original/Omicron BA.1 or tozinameran/riltozinameran) was authorized as a booster for use in the UK. The same... 236 KB (19,398 words) - 05:58, 20 April 2024 |
 | in vitro transcription and is found in the SARS-CoV-2 mRNA vaccines tozinameran (Pfizer–BioNTech) and elasomeran (Moderna). N1-Methylpseudouridine is... 16 KB (1,510 words) - 19:37, 11 April 2024 |
selexipag (2015), latanoprostene bunod (2017), benzhydrocodone (2018), tozinameran (2020) and serdexmethylphenidate (2021). Prodrugs can be classified into... 12 KB (1,279 words) - 13:32, 23 February 2024 |
(e.g. alipogene tiparvovec) -meran for messenger RNA products (e.g. tozinameran) The term stem is not used consistently in linguistics. It has been defined... 17 KB (1,618 words) - 09:49, 26 November 2023 |
mRNA vaccine (0.05 mg per dose) that contains the active ingredient tozinameran. Pfizer–BioNTech COVID-19 vaccine nanoparticle ingredients ALC-0315 1... 2 KB (205 words) - 21:16, 8 February 2024 |
the European Medicines Agency (EMA), and the first COVID-19 vaccine, Tozinameran from Pfizer/BioNTech, was approved on 21 December 2020. In concert with... 6 KB (389 words) - 21:24, 27 August 2023 |
Administration (FDA) for the mRNA vaccine BNT162b2 (active ingredient tozinameran) on 20 November 2020. On 2 December 2020, the United Kingdom's Medicines... 192 KB (22,024 words) - 10:32, 9 May 2024 |
 | and chief medical officer of BioNTech. Her team developed BNT162b2 (tozinameran (INN)), commonly known as the Pfizer–BioNTech COVID-19 vaccine. 2020:... 193 KB (19,007 words) - 00:38, 12 April 2024 |
drugs. After October 2020, the company produced the COVID-19 vaccine tozinameran at the Brenna site on behalf of Biontech. Since the end of April 2021... 4 KB (299 words) - 03:24, 28 March 2024 |
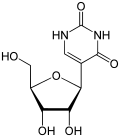 | the SARS-CoV2 vaccine from BioNTech/Pfizer, also known as BNT162b2, tozinameran or Comirnaty, all U's have been substituted with N1-methylpseudouridine... 24 KB (2,806 words) - 06:49, 1 April 2024 |
 | more developed protective levels of antibodies after vaccination with tozinameran. The antibody binds to the cell surface protein CD20. CD20 is widely... 47 KB (4,277 words) - 19:46, 30 March 2024 |
totrombopag choline (USAN) tovorafenib (INN) tozadenant (USAN) tozalinone (INN) tozasertib (USAN, INN) tozinameran (INN) TPN Electrolytes TPN Suspension... 7 KB (373 words) - 02:05, 25 April 2024 |
and MERS. The effect was not shown in phase III clinical trials for Tozinameran or for the Moderna vaccine. Acosta, Patricio L.; Caballero, Mauricio... 5 KB (462 words) - 16:13, 4 May 2023 |
release). 31 December 2020. Retrieved 6 January 2021. "WHO recommendation Tozinameran – COVID-19 mRNA vaccine (nucleoside modified) – Comirnaty". World Health... 318 KB (19,351 words) - 20:00, 21 April 2024 |
BioNTech's COVID-19 vaccine, Comirnaty, and its nonproprietary name, tozinameran. Comirnaty was the first COVID-19 vaccine brand name approved by any... 10 KB (885 words) - 15:16, 23 April 2024 |
 | original on 2022-09-30. Retrieved 2022-09-30. Health Canada. "Comirnaty (tozinameran)". COVID-19 vaccines and treatments portal. Archived from the original... 25 KB (2,035 words) - 02:12, 6 May 2024 |
 | the World Health Organization granted emergency use listing for the Tozinameran – COVID-19 mRNA vaccine (nucleoside modified) – Comirnaty. In January... 118 KB (12,634 words) - 14:17, 25 January 2024 |
genetic vaccines approved for use in humans include the RNA vaccines tozinameran and mRNA-1273, the DNA vaccine ZyCoV-D as well as the viral vectors AZD1222... 8 KB (1,186 words) - 19:47, 27 October 2022 |
However, this female patient was only able to have her first dose of Tozinameran Pfizer–BioNTech vaccine on January 13, 2021. DOH reiterated that vaccine... 162 KB (17,928 words) - 18:58, 12 February 2024 |






