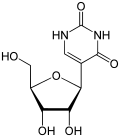
Pfizer–BioNTech COVID-19 vaccine (redirect from Tozinameran)
version of the bivalent vaccine (Comirnaty Original/Omicron BA.1 or tozinameran/riltozinameran) was authorized as a booster for use in the UK. The same...
260 KB (22,082 words) - 05:55, 13 September 2024
in vitro transcription and is found in the SARS-CoV-2 mRNA vaccines tozinameran (Pfizer–BioNTech) and elasomeran (Moderna). N1-Methylpseudouridine is...
16 KB (1,510 words) - 08:52, 2 September 2024
the European Medicines Agency (EMA), and the first COVID-19 vaccine, Tozinameran from Pfizer/BioNTech, was approved on 21 December 2020. In concert with...
6 KB (433 words) - 20:34, 16 May 2024
selexipag (2015), latanoprostene bunod (2017), benzhydrocodone (2018), tozinameran (2020) and serdexmethylphenidate (2021). Prodrugs can be classified into...
12 KB (1,281 words) - 06:14, 9 September 2024
(e.g. alipogene tiparvovec) -meran for messenger RNA products (e.g. tozinameran) The term stem is not used consistently in linguistics. It has been defined...
17 KB (1,650 words) - 13:04, 18 June 2024
Pharma Proprietary medications Pfizer Generic Viagra, Comirnaty (tozinameran, co-op with BioNTech) GlaxoSmithKline Amoxil (amoxicillin), Ventolin (salbutamol)...
41 KB (3,595 words) - 03:08, 12 July 2024
mRNA vaccine (0.05 mg per dose) that contains the active ingredient tozinameran. Pfizer–BioNTech COVID-19 vaccine nanoparticle ingredients ALC-0315 1...
2 KB (205 words) - 21:16, 8 February 2024
Administration (FDA) for the mRNA vaccine BNT162b2 (active ingredient tozinameran) on 20 November 2020. On 2 December 2020, the United Kingdom's Medicines...
202 KB (22,914 words) - 18:39, 23 September 2024
Administration (FDA) for the mRNA vaccine BNT162b2 (active ingredient tozinameran) on 20 November 2020. On 2 December 2020, the United Kingdom's Medicines...
122 KB (11,033 words) - 22:07, 17 July 2024
drugs. After October 2020, the company produced the COVID-19 vaccine tozinameran at the Brenna site on behalf of Biontech. Since the end of April 2021...
4 KB (300 words) - 08:38, 25 May 2024
1074/JBC.M310175200. ISSN 0021-9258. PMID 14729660. Wikidata Q34290592. Tozinameran – COVID-19 vaccine from Pfizer BioNTech, sold under the brand name Comirnaty...
43 KB (4,593 words) - 00:29, 24 September 2024
the SARS-CoV2 vaccine from BioNTech/Pfizer, also known as BNT162b2, tozinameran or Comirnaty, all U's have been substituted with N1-methylpseudouridine...
24 KB (2,835 words) - 06:46, 28 July 2024
and MERS. The effect was not shown in phase III clinical trials for Tozinameran or for the Moderna vaccine. Acosta, Patricio L.; Caballero, Mauricio...
5 KB (462 words) - 16:13, 4 May 2023
release). 31 December 2020. Retrieved 6 January 2021. "WHO recommendation Tozinameran – COVID-19 mRNA vaccine (nucleoside modified) – Comirnaty". World Health...
322 KB (19,558 words) - 03:33, 16 September 2024
totrombopag choline (USAN) tovorafenib (INN) tozadenant (USAN) tozalinone (INN) tozasertib (USAN, INN) tozinameran (INN) TPN Electrolytes TPN Suspension...
7 KB (373 words) - 02:05, 25 April 2024
such as Moderna's mRNA-1273, the Janssen vaccine, and Pfizer–BioNTech's Tozinameran, which all require both specialized manufacturing facilities and also...
14 KB (1,165 words) - 21:02, 26 August 2024
and chief medical officer of BioNTech. Her team developed BNT162b2 (tozinameran (INN)), commonly known as the Pfizer–BioNTech COVID-19 vaccine. 2020:...
194 KB (19,174 words) - 01:21, 9 September 2024
BioNTech's COVID-19 vaccine, Comirnaty, and its nonproprietary name, tozinameran. Comirnaty was the first COVID-19 vaccine brand name approved by any...
10 KB (855 words) - 14:53, 22 July 2024
the World Health Organization granted emergency use listing for the Tozinameran – COVID-19 mRNA vaccine (nucleoside modified) – Comirnaty. In January...
119 KB (12,643 words) - 00:40, 13 September 2024
Retrieved 2022-09-30. Health Canada (9 December 2020). "Comirnaty (tozinameran)". COVID-19 vaccines and treatments portal. Archived from the original...
25 KB (2,045 words) - 06:35, 23 September 2024
However, this female patient was only able to have her first dose of Tozinameran Pfizer–BioNTech vaccine on January 13, 2021. DOH reiterated that vaccine...
162 KB (17,926 words) - 06:23, 12 July 2024
infections after the first dose of the Pfizer–BioNTech vaccine (tozinameran) or placebo in a double-blind clinical trial (red: placebo; blue: tozinameran)...
95 KB (18,965 words) - 23:35, 15 September 2024
genetic vaccines approved for use in humans include the RNA vaccines tozinameran and mRNA-1273, the DNA vaccine ZyCoV-D as well as the viral vectors AZD1222...
8 KB (1,186 words) - 05:09, 11 June 2024
Health announced the purchase of an additional 100 million doses of the Tozinameran vaccine from Pfizer/BioNTech. With this, the company must deliver throughout...
118 KB (9,249 words) - 18:41, 22 May 2024
Sainte-Justine (in French). Retrieved 2022-02-05. Health Canada. "Comirnaty (tozinameran)". COVID-19 vaccines and treatments portal. Archived from the original...
20 KB (1,701 words) - 14:47, 22 May 2024