Fludrocortisone
 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Astonin, Astonin-H, others |
| Other names | StC-1400; 9α-Fluorohydrocortisone; 9α-Fluorocortisol; 9α-Fluoro-17α-hydroxycorticosterone; 9α-Fluoro-11β,17α,21-trihydroxypregn-4-ene-3,20-dione |
| AHFS/Drugs.com | Monograph |
| Pregnancy category |
|
| Routes of administration | By mouth |
| Drug class | Corticosteroid; glucocorticoid; mineralocorticoid |
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Protein binding | High |
| Metabolism | Liver |
| Elimination half-life | 3.5 hours |
| Identifiers | |
| |
| CAS Number |
|
| PubChem CID | |
| PubChem SID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| PDB ligand | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.004.395 |
| Chemical and physical data | |
| Formula | C21H29FO5 |
| Molar mass | 380.456 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
 | |
| Clinical data | |
|---|---|
| Trade names | Cortineff, Florinef, Florinefe, Fludrocortison, others |
| Other names | Fluorohydrocortisone acetate; 9α-Fluorohydrocortisone 21-acetate; 9α-Fluoro-17α-hydroxycorticosterone 21-acetate; 9α-Fluoro-11β,17α,21-trihydroxypregn-4-ene-3,20-dione 21-acetate |
| Routes of administration | By mouth |
| Drug class | Corticosteroid; glucocorticoid; mineralocorticoid |
| Pharmacokinetic data | |
| Metabolism | Liver |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.004.395 |
| Chemical and physical data | |
| Formula | C23H31FO6 |
| Molar mass | 422.493 g·mol−1 |
| 3D model (JSmol) | |
| Melting point | 260 to 262 °C (500 to 504 °F) (dec.) |
| |
| |
Fludrocortisone, sold under the brand name Florinef, among others, is a corticosteroid used to treat adrenogenital syndrome, postural hypotension, and adrenal insufficiency.[1][2][3] In adrenal insufficiency, it is generally taken together with hydrocortisone.[3] Fludrocortisone is taken by mouth[3] and is most commonly used in its acetate form.[4]
Common side effects of fludrocortisone include high blood pressure, swelling, heart failure, and low blood potassium.[3] Other serious side effects can include low immune-system function, cataracts, muscle weakness, and mood changes.[3] Whether use of fludrocortisone during pregnancy is safe for the fetus is unknown.[5] Fludrocortisone is mostly a mineralocorticoid, but it also has glucocorticoid effects.[3]
Fludrocortisone was patented in 1953.[6] It is on the World Health Organization's List of Essential Medicines.[7]
Medical uses[edit]
Fludrocortisone has been used in the treatment of cerebral salt-wasting syndrome.[8] It is used primarily to replace the missing hormone aldosterone in various forms of adrenal insufficiency such as Addison's disease and the classic salt-wasting (21-hydroxylase deficiency) form of congenital adrenal hyperplasia. Due to its effects on increasing Na+ levels, and therefore blood volume, fludrocortisone is the first-line of treatment for orthostatic intolerance, and postural orthostatic tachycardia syndrome (POTS).[9] It can be used to treat low blood pressure.[10]
Fludrocortisone is also a confirmation test for diagnosing Conn's syndrome (aldosterone-producing adrenal adenoma), the fludrocortisone suppression test. Loading the patient with fludrocortisone would suppress serum aldosterone level in a normal patient, whereas the level would remain elevated in a Conn's patient. The fludrocortisone suppression test is an alternative to the NaCl challenge (which would use normal saline or salt tablets).[medical citation needed]
Side effects[edit]
Use of Fludrocortisone can lead to one or more of the following side effects:[11]
- Sodium and water retention
- Swelling due to fluid retention (edema)
- High blood pressure (hypertension)
- Headache
- Low blood potassium level (hypokalemia)
- Muscle weakness
- Fatigue
- Increased susceptibility to infection
- Impaired wound healing
- Increased sweating
- Increased hair growth (hirsutism)
- Thinning of skin and stretch marks
- Disturbances of the gut such as indigestion (dyspepsia), distention of the abdomen and ulceration (peptic ulcer)
- Decreased bone density and increased risk of fractures of the bones
- Difficulty in sleeping (insomnia)
- Depression
- Weight gain
- Raised blood sugar level
- Changes to the menstrual cycle
- Partial loss of vision due to opacity in the lens of the eye (cataracts)
- Raised pressure in the eye (glaucoma)
- Increased pressure in the skull (intracranial pressure)
Pharmacology[edit]
Fludrocortisone is a corticosteroid and acts as a powerful mineralocorticoid, along with some additional but comparatively very weak glucocorticoid activity.[12] Relative to cortisol, it is said to have 10 times the glucocorticoid potency but 250 to 800 times the mineralocorticoid potency.[12][13] Fludrocortisone acetate is a prodrug of fludrocortisone, which is the active form of the drug.[14]
Plasma renin, sodium, and potassium are checked through blood tests to verify that the correct dosage is reached.[medical citation needed]
Chemistry[edit]
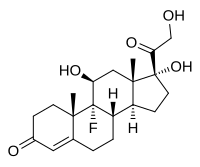
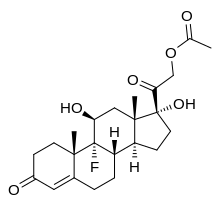
Fludrocortisone, also known as 9α-fluorocortisol (9α-fluorohydrocortisone) or as 9α-fluoro-11β,17α,21-trihydroxypregn-4-ene-3,20-dione, is a synthetic pregnane steroid and a halogenated derivative of cortisol (11β,17α,21-trihydroxypregn-4-ene-3,20-dione).[1][2] Specifically, it is a modification of cortisol with a fluorine atom substituted in place of one hydrogen atom at the C9α position.[1][2] Fluorine is a good bioisostere for hydrogen because it is similar in size, with the major difference being in its electronegativity. The acetate form of fludrocortisone, fludrocortisone acetate, is the C21 acetate ester of fludrocortisone,[1][2] and is hydrolyzed into fludrocortisone in the body.[14]
History[edit]
Fludrocortisone was described in the literature in 1953[15] and was introduced for medical use (as the acetate ester) in 1954.[13][16] It was the first synthetic corticosteroid to be marketed, and followed the introduction of cortisone in 1948 and hydrocortisone (cortisol) in 1951.[15][17] Fludrocortisone was also the first fluorine-containing pharmaceutical drug to be marketed.[18]
Society and culture[edit]
Generic name[edit]
Fludrocortisone is the generic name of fludrocortisone and its INN, USAN, BAN, DCF, and DCIT, whereas fludrocortisone acetate is the generic name of fludrocortisone acetate and its USP, BANM and JAN.[1][2][19]
Brand names[edit]
Fludrocortisone is marketed mainly under the brand names Astonin and Astonin-H, whereas the more widely used fludrocortisone acetate is sold mainly as Florinef, but also under several other brand names including Cortineff, Florinefe, and Fludrocortison.[2][19]
Availability[edit]
Fludrocortisone is marketed in Austria, Croatia, Denmark, Germany, Luxembourg, Romania, and Spain, whereas fludrocortisone acetate is more widely available throughout the world and is marketed in the United States, Canada, the United Kingdom, various other European countries, Australia, Japan, China, Brazil, and many other countries.[2][19]
References[edit]
- ^ a b c d e Elks J (14 November 2014). The Dictionary of Drugs: Chemical Data: Chemical Data, Structures and Bibliographies. Springer. pp. 558–. ISBN 978-1-4757-2085-3. Archived from the original on 5 November 2017.
- ^ a b c d e f g Index Nominum 2000: International Drug Directory. Taylor & Francis. 2000. pp. 450–. ISBN 978-3-88763-075-1. Archived from the original on 2017-11-05.
- ^ a b c d e f "Fludrocortisone Acetate". The American Society of Health-System Pharmacists. Archived from the original on 5 July 2017. Retrieved 8 December 2016.
- ^ Day RO, Furst DE, van Riel PL, Bresnihan B, eds. (30 May 2010). "Medicinal Chemistry of the Disease Modifying Antirheumatic Drugs". Antirheumatic Therapy: Actions and Outcomes. Springer Science & Business Media. pp. 21–. ISBN 978-3-7643-7726-7. Archived from the original on 5 November 2017.
- ^ "Fludrocortisone Use During Pregnancy | Drugs.com". www.drugs.com. Archived from the original on 24 December 2016. Retrieved 24 December 2016.
- ^ Fischer J, Ganellin CR (2006). Analogue-based Drug Discovery. John Wiley & Sons. p. 484. ISBN 9783527607495. Archived from the original on 2017-11-05.
- ^ World Health Organization (2019). World Health Organization model list of essential medicines: 21st list 2019. Geneva: World Health Organization. hdl:10665/325771. WHO/MVP/EMP/IAU/2019.06. License: CC BY-NC-SA 3.0 IGO.
- ^ Taplin CE, Cowell CT, Silink M, Ambler GR (December 2006). "Fludrocortisone therapy in cerebral salt wasting". Pediatrics. 118 (6): e1904–e1908. doi:10.1542/peds.2006-0702. PMID 17101713. S2CID 28871495.
- ^ Freitas J, Santos R, Azevedo E, Costa O, Carvalho M, de Freitas AF (October 2000). "Clinical improvement in patients with orthostatic intolerance after treatment with bisoprolol and fludrocortisone". Clinical Autonomic Research. 10 (5): 293–299. doi:10.1007/BF02281112. PMID 11198485. S2CID 20843222.
- ^ Veazie S, Peterson K, Ansari Y, Chung KA, Gibbons CH, Raj SR, Helfand M (May 2021). "Fludrocortisone for orthostatic hypotension". The Cochrane Database of Systematic Reviews. 2021 (5): CD012868. doi:10.1002/14651858.CD012868.pub2. PMC 8128337. PMID 34000076.
- ^ "Fludrocortisone Oral: Uses, Side Effects, Interactions, Pictures, Warnings & Dosing - WebMD". www.webmd.com. Retrieved 2023-09-05.
- ^ a b De Groot LJ, Chrousos G, Dungan K, Feingold KR, Grossman A, Hershman JM, et al. (2000). "Glucocorticoid Therapy and Adrenal Suppression". In Feingold KR, Anawalt B, Blackman MR, Boyce A, Chrousos G, Corpas E, et al. (eds.). Endotext [Internet]. South Dartmouth (MA): MDText.com, Inc. PMID 25905379.
- ^ a b Lemke TL, Williams DA (2008). Foye's Principles of Medicinal Chemistry. Lippincott Williams & Wilkins. pp. 890–. ISBN 978-0-7817-6879-5. Archived from the original on 2017-11-05.
- ^ a b Polito A, Hamitouche N, Ribot M, Polito A, Laviolle B, Bellissant E, et al. (December 2016). "Pharmacokinetics of oral fludrocortisone in septic shock". British Journal of Clinical Pharmacology. 82 (6): 1509–1516. doi:10.1111/bcp.13065. PMC 5099539. PMID 27416887.
- ^ a b Calvert DN (August 1962). "Anti-inflammatory steroids". Wisconsin Medical Journal. 61: 403–404. PMID 13875857.
- ^ William Andrew Publishing (22 October 2013). Pharmaceutical Manufacturing Encyclopedia, 3rd Edition. Elsevier. pp. 1642–. ISBN 978-0-8155-1856-3. Archived from the original on 5 November 2017.
- ^ Khan MO, Park KK, Lee HJ (2005). "Antedrugs: an approach to safer drugs". Current Medicinal Chemistry. 12 (19): 2227–2239. doi:10.2174/0929867054864840. PMID 16178782.
- ^ Walker MC, Chang MC (September 2014). "Natural and engineered biosynthesis of fluorinated natural products". Chemical Society Reviews. 43 (18): 6527–6536. doi:10.1039/c4cs00027g. PMID 24776946. S2CID 205904152.
- ^ a b c "Fludrocortisone Uses, Side Effects & Warnings". Archived from the original on 2015-05-13. Retrieved 2017-07-16.
External links[edit]
- "Fludrocortisone". Drug Information Portal. U.S. National Library of Medicine.


 French
French Deutsch
Deutsch